A blog that publishes updates and open access scientific papers about allergy, asthma and immunology. Editor: Juan Carlos Ivancevich, MD. Specialist in Allergy & Immunology
December 9, 2020
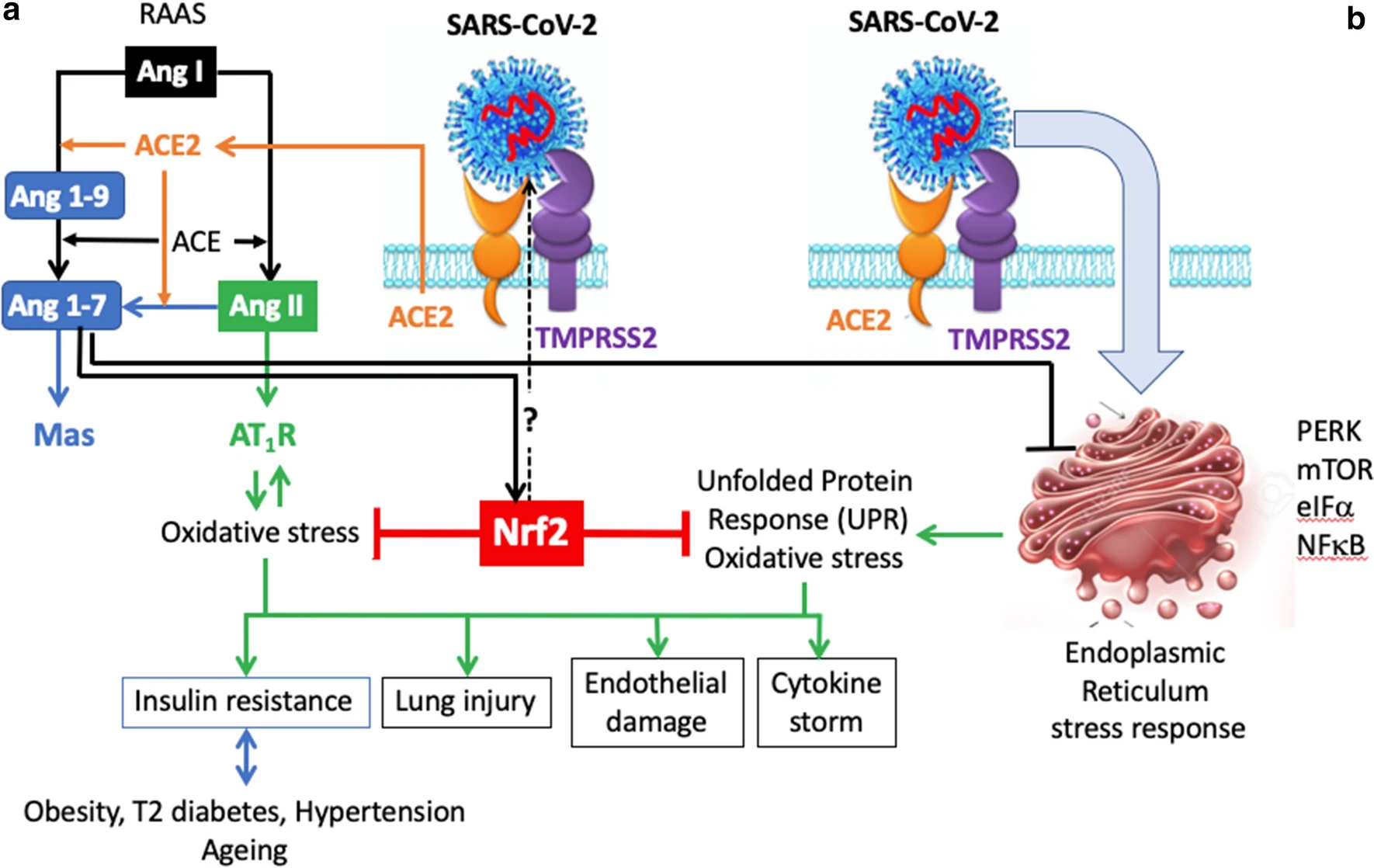
Nrf2-interacting nutrients and COVID-19: time for research to develop adaptation strategies
December 7, 2020
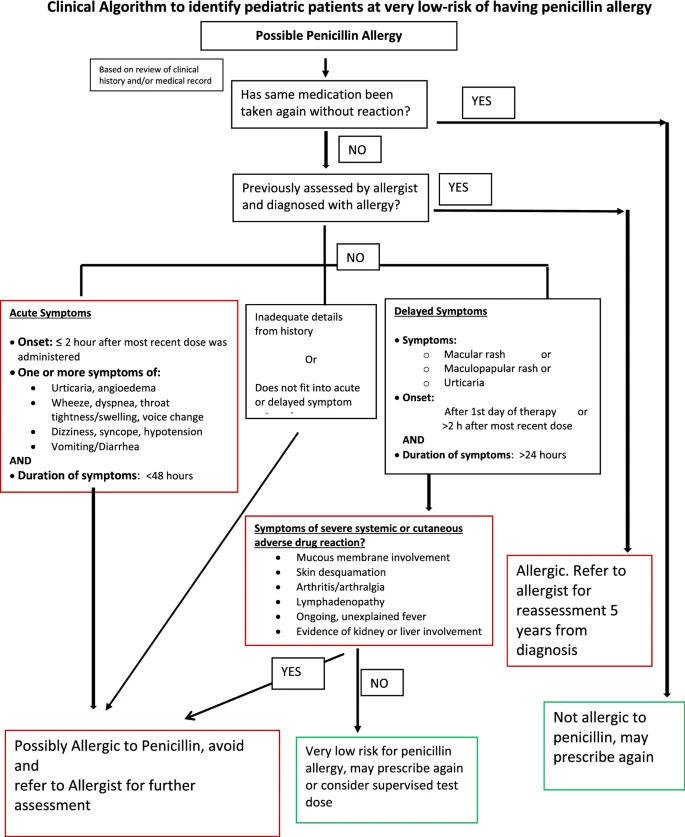
First pediatric electronic algorithm to stratify risk of penicillin allergy
- Letter to the editor
- Open Access
Allergy, Asthma & Clinical Immunology
- Hannah Roberts,
- Lianne Soller,
- Karen Ng,
- Edmond S. Chan,
- Ashley Roberts,
- Kristopher Kang,
- Kyla J. Hildebrand &
- Tiffany Wong
Allergy, Asthma & Clinical Immunology volume 16, Article number: 103 (2020)
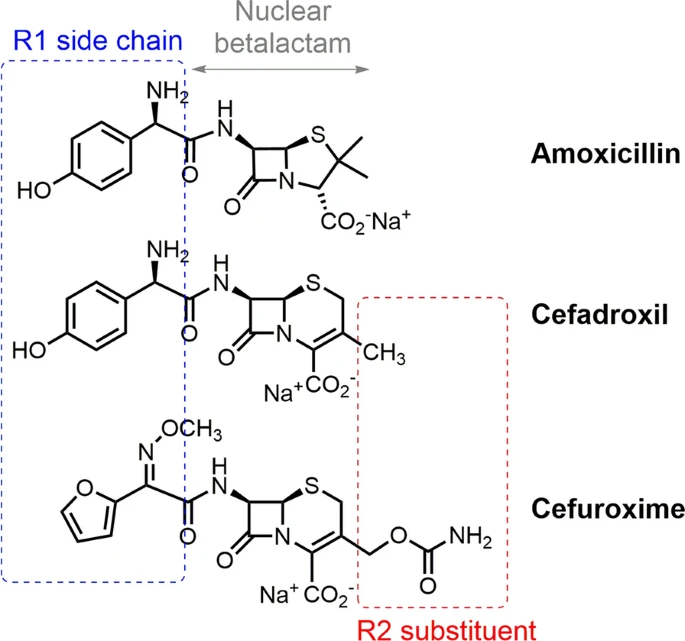
Penicillin and cephalosporin cross-reactivity: role of side chain and synthetic cefadroxil epitopes
- Research
- Open Access
Clinical and Translational Allergy
- Gador Bogas,
- Cristobalina Mayorga,
- Ángela Martín-Serrano,
- Rubén Fernández-Santamaría,
- Isabel M. Jiménez-Sánchez,
- Adriana Ariza,
- Esther Barrionuevo,
- Teresa Posadas,
- María Salas,
- Tahía Diana Fernández,
- María José Torres &
- María Isabel Montañez
Clinical and Translational Allergy volume 10, Article number: 57 (2020)
Background
Analysis of cross-reactivity is necessary for prescribing safe cephalosporins for penicillin allergic patients. Amoxicillin (AX) is the betalactam most often involved in immediate hypersensitivity reactions (IHRs), and cefadroxil (CX) the most likely cephalosporin to cross-react with AX, since they share the same R1 side chain, unlike cefuroxime (CO), with a structurally different R1. We aimed to analyse cross-reactivity with CX and CO in patients with confirmed IHRs to AX, including sIgE recognition to AX, CX, CO, and novel synthetic determinants of CX.
November 25, 2020
World Allergy Organization Journal Volume 13, Issue 11 , November 2020
|
|
|
|
November 18, 2020
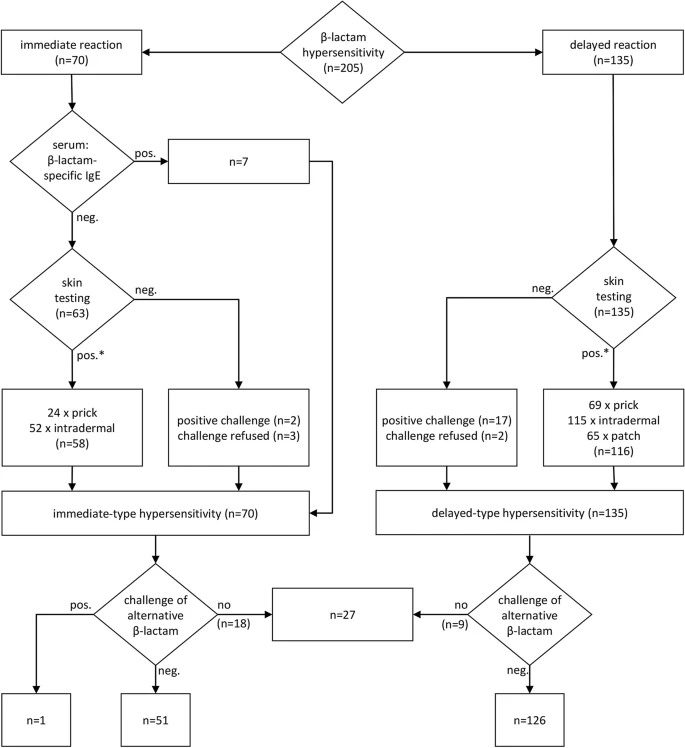
Predominant patterns of β-lactam hypersensitivity in a single German Allergy Center: exanthem induced by aminopenicillins, anaphylaxis by cephalosporins
- Research
- Open Access
Allergy, Asthma & Clinical Immunology
Allergy, Asthma & Clinical Immunology volume 16, Article number: 102 (2020)
November 16, 2020
Safety of measles, mumps, and rubella vaccine in egg allergy: in vivo and in vitro management
- Case Report
- Open Access
- Stefania Magistà,
- Marcello Albanesi,
- Nada Chaoul,
- Danilo Di Bona,
- Elisabetta Di Leo,
- Eustachio Nettis,
- Maria Filomena Caiaffa &
- Luigi Macchia
Clinical and Molecular Allergy volume 18, Article number: 21 (2020)
Egg allergy is the second most prevalent form of food allergy in childhood. In spite of the evidence accumulated, inoculating egg allergy children with attenuated vaccines grown on chick embryo cell cultures, such as the measles, mumps, and rubella (MMR) vaccine, is regarded (erroneously) as potentially dangerous or even anaphylactogenic, by many. An issue perceived as particularly conflicting also by Health Professionals.
November 11, 2020
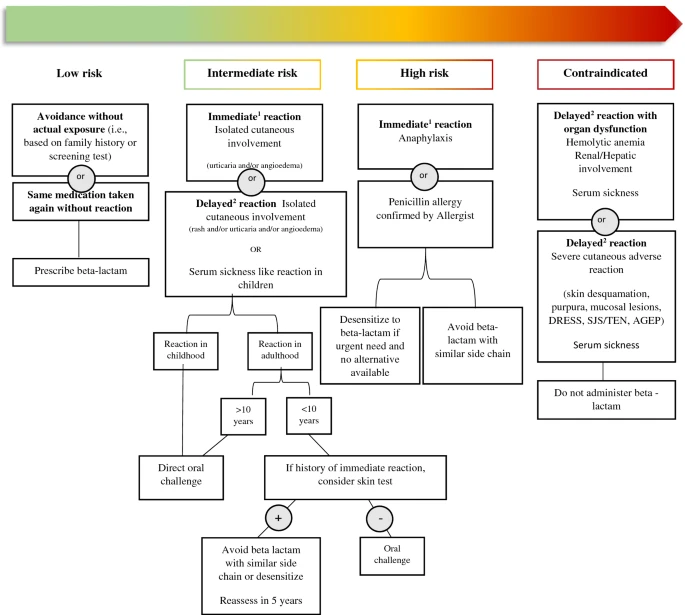
Practical guide for evaluation and management of beta-lactam allergy: position statement from the Canadian Society of Allergy and Clinical Immunology
- Allergy, Asthma & Clinical ImmunologyReview
- Open Access
Abstract
The vast majority of individuals labelled as allergic are not deemed truly allergic upon appropriate assessment by an allergist. A label of beta-lactam allergy carries important risks for individual and public health. This article provides an overview of beta-lactam allergy, implications of erroneous beta-lactam allergy labels and the impact that can be provided by structured allergy assessment. We provide recommendations on how to stratify risk of beta-lactam allergy, beta lactam challenge protocols as well as management of patients at high risk of beta-lactam allergy.
November 4, 2020
A single centre retrospective study of systemic reactions to subcutaneous immunotherapy
- Research
- Open Access
Allergy, Asthma & Clinical Immunology
Allergy, Asthma & Clinical Immunology volume 16, Article number: 93 (2020)
Abstract
Background
Subcutaneous immunotherapy (SCIT) is the standard approach for treating patients with sensitizations to aeroallergens. However, immunotherapy can trigger severe systemic reactions if delivered inappropriately or to high risk patients. We sought to characterize and quantify SCIT systemic reactions requiring epinephrine administration during a 6-year period in a Canadian setting following the recommendations for components and dosages published in the 2010 Canadian Society of Allergy and Clinical Immunology (CSACI) Immunotherapy Manual.