Abstract
In recent years, specialized tests have been developed, such as Impulse Oscillometry (IOS) and Multiple Breath Nitrogen Washout (MBNW) tests, which have been deemed more accurate in detecting SAD than conventional spirometry.
A blog that publishes updates and open access scientific papers about allergy, asthma and immunology. Editor: Juan Carlos Ivancevich, MD. Specialist in Allergy & Immunology
In recent years, specialized tests have been developed, such as Impulse Oscillometry (IOS) and Multiple Breath Nitrogen Washout (MBNW) tests, which have been deemed more accurate in detecting SAD than conventional spirometry.
Sucheta Sharma, Nishat Tasnim, Kuchalambal Agadi,Ummul Asfeen, and Jatin Kanda
Abstract
Asthma is a non-communicable and long-term condition affecting children and adults. The air passages in the lungs become narrow due to inflammation and tightening of the muscles around the small airways. Symptoms of asthma are intermittent and include cough, wheeze, shortness of breath, and chest tightness. Asthma is very often underdiagnosed and under-treated in many regions, especially in developing countries. While many studies show that viral infections can precipitate asthmatic attacks, very few studies have been conducted to see if history or current asthmatic attack increases the risk of viral infections. Our study aims to determine the predisposition of asthmatics to develop various viral infections and susceptibility toward certain viruses that cause upper respiratory tract infections.
We performed a literature review of both published and unpublished articles. We included case reports, case series, reviews, clinical trials, cohort, and case-control studies, written only in English. Commentaries, letters to editors, and book chapters were excluded. Our initial search yielded 948 articles, of which 826 were rejected either because they were irrelevant or because they did not meet our inclusion criteria. We finally screened 122 abstracts and identified 24 relevant articles.
People with a history of asthma have an abnormal innate immune response, making them potentially slower in clearing the infection and susceptible to both infections and virus-induced cell cytotoxicity.
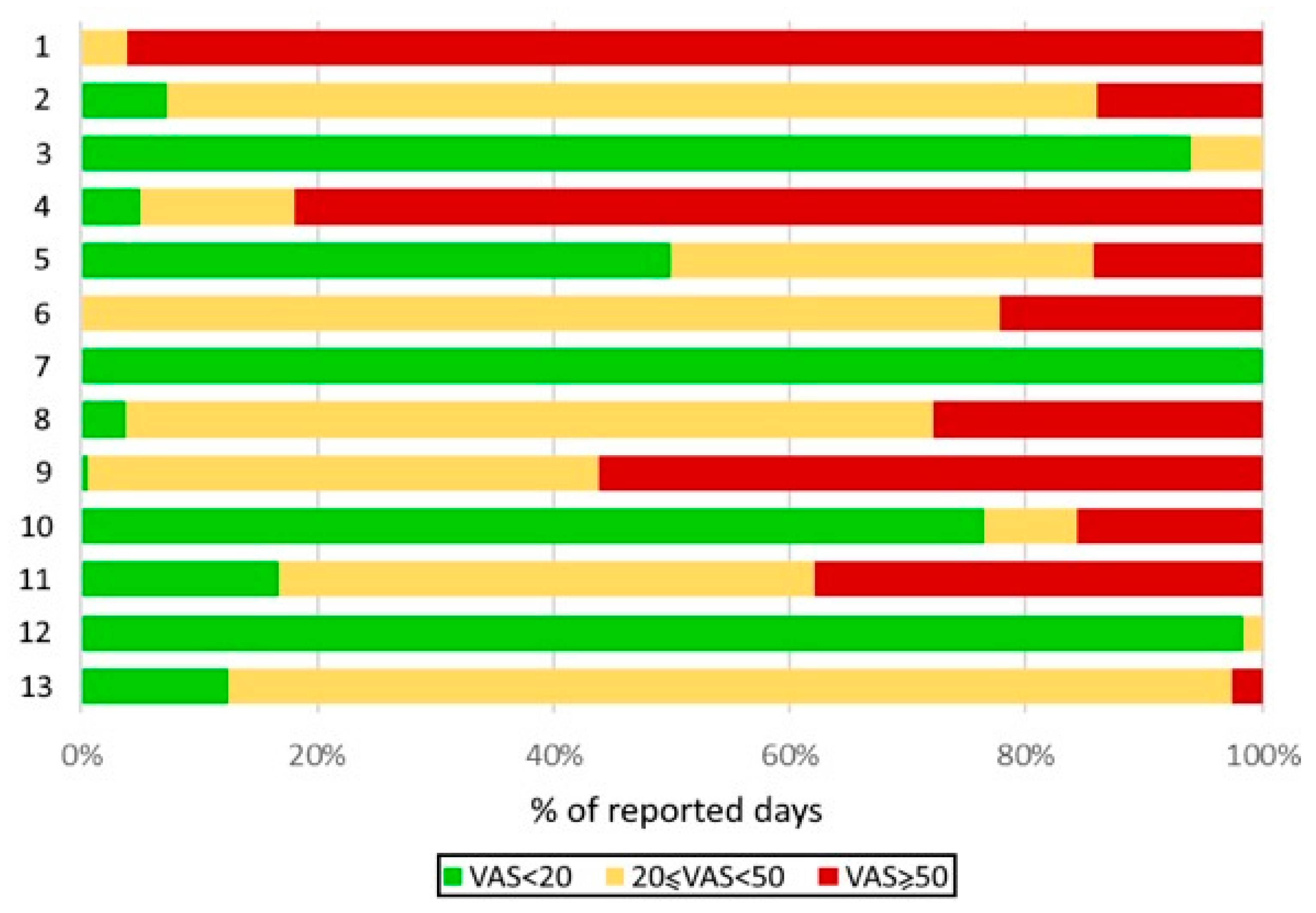 |
Distribution of well-controlled, moderately controlled, and poorly controlled days by patient, as assessed by the visual analogue scale (VAS) quantifying the severity of asthma symptoms. |
Hypersensitivity pneumonitis (HP) is an interstitial lung disease characterized by antigen-triggered neutrophilic exacerbations. Although CD4+ T cells are sufficient for HP pathogenesis, this never translated into efficient T cell-specific therapies. Increasing evidence shows that B cells also play decisive roles in HP. Here, we aimed to further define the respective contributions of B and T cells in subacute experimental HP.
The lymphocyte transformation test (LTT) is an in vitro assay used to diagnose drug induced hypersensitivity reactions by detecting the activation and expansion of drug-specific memory T cells to the suspected implicated drug. Traditionally radiolabelled thymidine (3H-thymidine) has been used but requires the handling and disposal of radioactive materials.
Allergy, Asthma & Clinical Immunology volume 18, Article number: 89 (2022)
Dupilumab-induced ocular surface disease (DIOSD) has been reported in patients with atopic dermatitis treated with dupilumab, and has been recognized as an adverse event of dupilumab. Our objective was to describe two cases of DIOSD with alterations in eotaxin-2 and interleukin (IL)-8 messenger ribonucleic acid (mRNA) expression on the ocular surface.
In the ocular surface test, specimens were collected from the patient's ocular surface, and eotaxin-2 and IL-8 mRNA levels in the specimens were measured using real-time polymerase chain reaction. The clinical score of ocular surface findings was quantified using a 5-5-5 exacerbation grading scale for allergic conjunctivitis. The first case was of a 27-year-old man who developed DIOSD 3 months after starting treatment with dupilumab injection for atopic dermatitis.
Immunoglobulin replacement therapy is the standard-of-care treatment for patients with primary immunodeficiency diseases who have impaired antibody production and function. Clinicians and patients may consider intravenous immunoglobulin (IVIG) or subcutaneous immunoglobulin (SCIG) options, and each route may offer different benefits for the individual. IVIG requires fewer infusion sites and less frequent infusions than some formulations of SCIG. However, SCIG does not require venous access, is associated with fewer systemic adverse infusion reactions than IVIG, and can independently be self-administered at home.