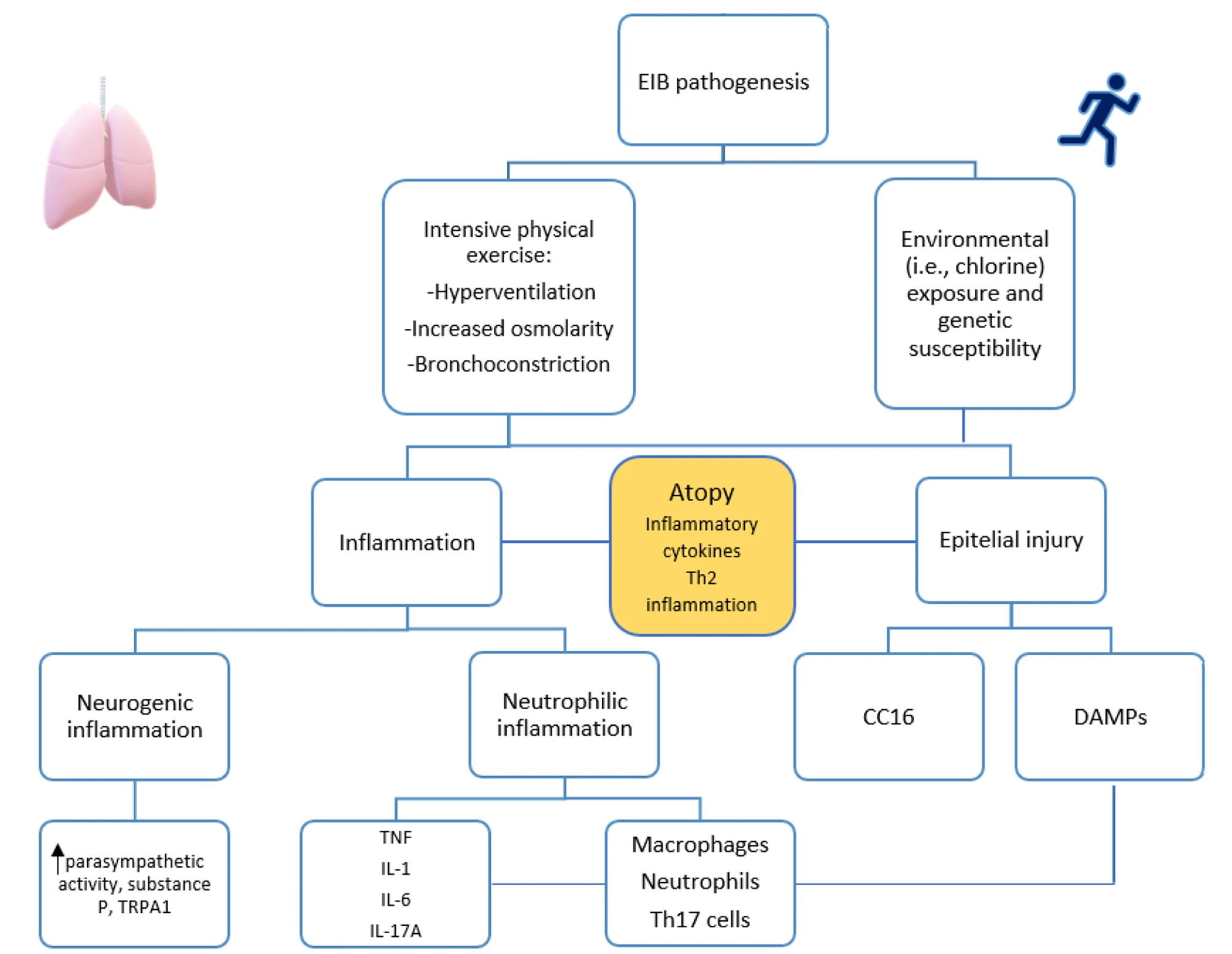
ABSTRACT
BACKGROUND AND OBJECTIVES:
Respiratory viral infections increase risk of asthma in infants and children. Infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus can cause severe lung inflammation and prolonged respiratory symptoms. We sought to determine whether SARS-CoV-2 infection modified pediatric incident asthma risk.
METHODS:
This retrospective cohort study examined children ages 1 to 16 within the Children’s Hospital of Philadelphia Care Network who received polymerase chain reaction (PCR) testing for SARS-CoV-2 between March 1, 2020 and February 28, 2021. Multivariable Cox regression models assessed the hazard ratio of new asthma diagnosis between SARS-CoV-2 PCR positive and SARS-CoV-2 PCR negative groups within an 18-month observation window. Models were adjusted for demographic characteristics, socioeconomic variables, and atopic comorbidities.
RESULTS:
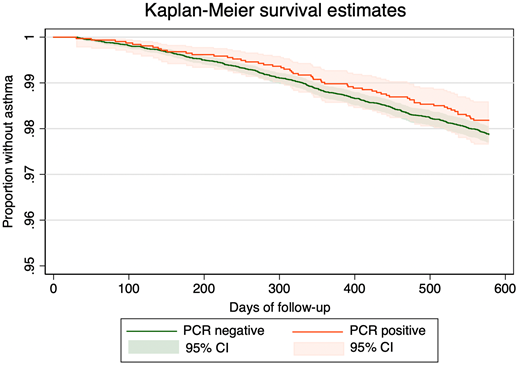 |
| Kaplan-Meier survival curves comparing adjusted asthma-free survival between SARS-CoV-2 positive and SARS-CoV-2 negative groups. |