- Allergy, Asthma & Clinical Immunology
- Research
- Open Access
Abstract
Background
Epinephrine is a lifesaving medication in the treatment of anaphylaxis. Epinephrine auto-injectors are the preferred method of epinephrine administration, but are not universally available or affordable.
Little is known about the effects on epinephrine when it is drawn up in advance and stored as prefilled syringes.
Objective
To study the stability and sterility of epinephrine when stored in syringes.
Methods
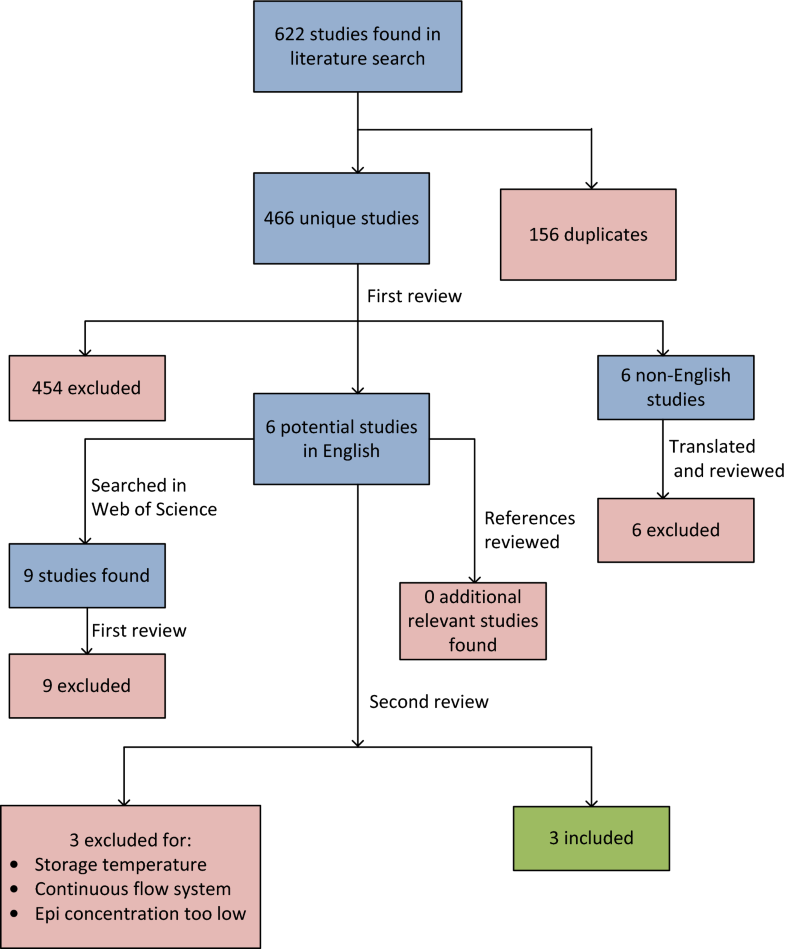
We searched Embase, Medline, and Web of Science in June 2016 for all studies of epinephrine stored in syringes in concentrations between 0.1 and 1 mg/mL that measured epinephrine stability and/or sterility over time, regardless of date published or language.
Results
Three studies were included, one testing two concentrations of epinephrine. Only one study tested epinephrine 1 mg/mL, the concentration clinically relevant for intramuscular use during anaphylaxis. Neither this study nor the one study testing 0.7 mg/mL epinephrine found significant degradation after 56 and 90 days, respectively. One of the two studies testing epinephrine at a concentration of 0.1 mg/mL found significant degradation by 14 days; the other found no degradation up to 168 days. Two studies tested for bacterial growth, with none detected after 28 and 90 days, respectively. One study tested for fungal growth, with none detected after 90 days.
Conclusions
Limited evidence suggests that syringes filled with 1 mg/mL epinephrine are stable and sterile for 90 days. More research is needed testing the duration of stability and sterility of prefilled syringes with the 1 mg/mL concentration most commonly used in anaphylaxis, testing more extensively in different storage conditions and across a wider range of marketed syringe brands.
No comments:
Post a Comment