Download PDF: US English
February 25, 2019 (Issue: 1566)
The FDA has approved a manually injected, single-dose, prefilled epinephrine syringe (Symjepi – Adamis/Sandoz) for emergency treatment of anaphylaxis. The new device is approved in 0.3- and 0.15-mg strengths for treatment of patients weighing ≥30 kg and 15 to 30 kg, respectively; only the 0.3-mg strength is currently available. According to Sandoz, Symjepi will be made available first to institutions and later to the retail market.

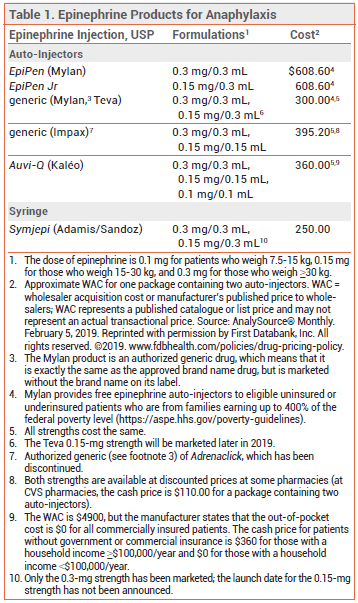
EPINEPHRINE AUTO-INJECTORS — EpiPen and EpiPen Jr, which are also available generically, have been used effectively for treatment of anaphylaxis for many years (see Table 1). A generic version of the Adrenaclick auto-injector (no longer manufactured) is also available; it is similar to EpiPenin size and functionality. Auvi-Q, which is the size of a thick credit card, provides visual signals and audio instructions, has an automatic needle retraction system, and appears to be more convenient to carry and easier to use than EpiPen.1 It is the only auto-injector available in a 0.1-mg strength for use in children weighing 7.5-15 kg.2 Because of differences in device design and instructions for use, these 3 auto-injectors are not considered interchangeable, and pharmacists cannot substitute one for another.

THE NEW DEVICE — The Symjepi syringe is stored in a plastic case that is 4.1" long, 1.6" wide, and 0.9" deep (EpiPen in its container is about 5.5" long and 1" in diameter and Auvi-Q is 3.5" long, 2" wide, and 0.5" thick). The solution should be inspected periodically through a window in the syringe; if the normally clear, colorless solution appears cloudy or has visible particulates or a pink or brown tint, the syringe should be discarded and replaced.
Like epinephrine auto-injectors, Symjepi is injected intramuscularly or subcutaneously into the anterolateral aspect of the thigh, through clothing if necessary. Before injection, the cap must be removed to expose the needle. Unlike epinephrine auto-injectors, Symjepi syringes require manual injection of the needle and depression of the plunger, which should be pushed down until it clicks; the syringe should then be held in place for two seconds. The single-dose syringe is intentionally overfilled; more than half of the solution will remain in the syringe after use. After the needle is withdrawn, the injection site should be massaged for ten seconds, and the safety guard should be extended manually until it covers the needle.
CLINICAL STUDY — In one study, the usability of a Symjepi device filled with saline was compared to that of an EpiPen trainer in 34 untrained adolescents 12-17 years old. All of the subjects tested both devices by simulating injection into a pad placed at the injection site; half of them tested Symjepi first and the other half tested EpiPen first. No use errors were reported with the Symjepi device compared to 4 (3 were accidental injection into the thumb of the user) with the EpiPen trainer.3
STABILITY — Epinephrine solutions have a variable, relatively short duration of stability. They should not be exposed to light or to extreme temperatures; they should not be stored in a refrigerator or the glove compartment of an automobile.4 As with epinephrine auto-injectors, Symjepi syringes should be replaced before their expiration date.
CONCLUSION — Symjepi, the new epinephrine single-dose, prefilled syringe for treatment of anaphylaxis, is priced lower than EpiPen and its generics. Unlike auto-injectors, Symjepi syringes require the user to manually inject the needle and push down the plunger, which may be difficult for some patients, particularly children.
- Auvi-Q epinephrine auto-injector returns. Med Lett Drugs Ther 2017; 59:33.
- In brief: Auvi-Q epinephrine auto-injector for infants and toddlers. Med Lett Drugs Ther 2018; 60:83.
- RB Moss et al. Human factors study in untrained adolescents comparing a recently approved single-dose epinephrine prefilled syringe with an approved autoinjector. Ann Allergy Asthma Immunol 2018; 120:540.
- P Lacwik et al. Single, short-time exposure to heat in a car during sunny day can decrease epinephrine concentration in autoinjectors: a real-life pilot study. J Allergy Clin Immunol Pract 2018 Nov 27 (epub).
No comments:
Post a Comment