Abstract
Introduction
To assess the safety and efficacy of an oral immunotherapy regimen in patients with allergy to lipid transfer proteins (LTPs).
Materials and methods
Prospective study of 24 patients allergic to LTP with positive skin test and a history of anaphylaxis. All patients underwent a desensitization protocol with commercial peach juice. Rising doses of peach juice were administered, starting with an initial dose of seven drops of a 1/1000 dilution and finishing with a dose of 5 ml at visit 17.
At visit 18, all patients performed an open challenge with whole juice at a cumulative dose of 200 ml. All adverse reactions occurring during the administration of the different doses were recorded. Levels of rPru p 3 in the juice were quantified.
Results
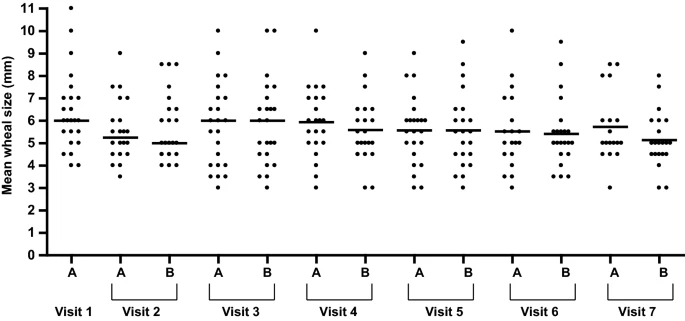 |
| Prick-by-prick test performed either with the juice just opened (a) or with the juice returned by the patient within 8-15 days of taking the doses at home (b) |
There were no severe reactions during the desensitization process in the 24 patients. Seven patients (29%) reported mild oral symptoms, and two patients (8%) had urticaria associated with co-factors (one due to exercise and another due to non-steroidal anti-inflammatory drugs). Nineteen patients were able to swallow 5 ml of juice and five withdrew from the study. In two pregnant patients the final challenge was not performed. In all, 17/24 patients were able to consume 200 ml peach juice without developing symptoms.
Conclusions
Oral immunotherapy with the regimen used in this study is an effective and safe short-term therapeutic option for patients with allergy to LTPs. Commercial peach juice appears to be suitable for this treatment.
x
No comments:
Post a Comment