Open Access
Original Research
Abstract
Introduction
Allergic asthma is a chronic inflammatory disease caused by immunoglobulin E (IgE)-mediated allergy. Omalizumab is a monoclonal anti-IgE antibody for the treatment of severe allergic asthma (SAA).
Objectives
The primary objective of the study was to assess asthma-related control in patients with SAA receiving omalizumab therapy. Secondary objectives included quality of life, treatment effectiveness, rate of severe exacerbations, and safety.
Methods
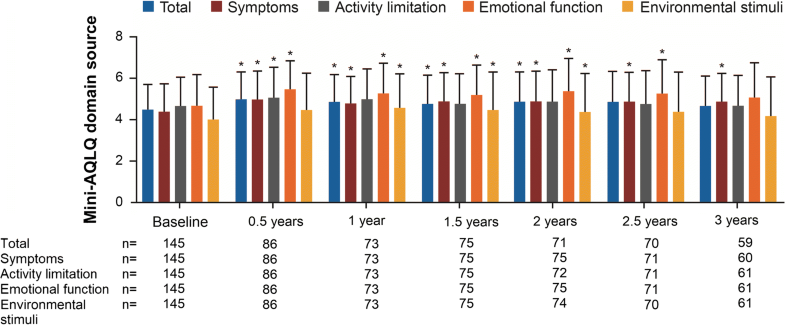
This was a prospective, multi-centre, non-interventional study to assess patient-related long-term outcomes of omalizumab treatment in Germany. This 3-year study enrolled patients aged ≥ 18 years with SAA. Asthma control was assessed using the asthma control questionnaire (ACQ-6) and physician-assessed global evaluation of treatment effectiveness (GETE). Exacerbations were recorded, and quality of life was assessed using the mini-asthma quality of life questionnaire (mini-AQLQ).
Results
Of 161 patients screened, 153 participated in this study. Most patients (92.2%) had been receiving prior omalizumab therapy for mean (SD) 2.9 (2.3) years. Omalizumab slightly decreased mean ACQ-6 score from 2.0 (1.22) at baseline to 1.7 (1.23) at the end of the 3-year treatment period [difference: –0.18 (1.07), P = 0.340]. Post-hoc analyses of ACQ-6 for the small number of treatment-naïve patients showed a decrease in mean (SD) ACQ-6 from 2.7 (1.08) at baseline to 1.4 (1.40) after 3 years of omalizumab treatment. Mini-AQLQ increased from 4.5 (1.26) at baseline to 4.7 (1.48) after 3 years [difference: 0.26 (1.35), P = 0.186]. GETE was reported as excellent or good for most patients (67.46–84.69%) and more than two-thirds had no severe exacerbation. There were no unexpected safety signals during the study period and no tachyphylaxis was observed.
Conclusions
In conclusion, despite most patients receiving prior omalizumab treatment for approximately 3 years, there was no decrease in effectiveness or safety over the subsequent 3 years during this study. This supports the long-term use of omalizumab in maintaining asthma control and quality of life.

No comments:
Post a Comment