Thomas Proctor,
Elodie Morrough,
Otto Fenske,
Sarah Allatt,
Stephen M. Hughes,
Vibha Sharma &
Peter D. Arkwright
Abstract
Background
Pollen and house dust mite (HDM) subcutaneous immunotherapy (SLIT) and pollen subcutaneous immunotherapy (SCIT) are effective therapies for children with allergic rhinoconjunctivitis (AR). There are no previous direct comparative studies investigating quality of life (QoL) of all three immunotherapy regimes. The aim of this study was to compare QoL and safety in children receiving these immunotherapies for AR.
Methods
Demographic characteristics, Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) and Visual Analogue (VAS) scores were assessed in 249 children undergoing HDM and pollen immunotherapy at a UK specialist paediatric centre between 2007 and 2019.
Results
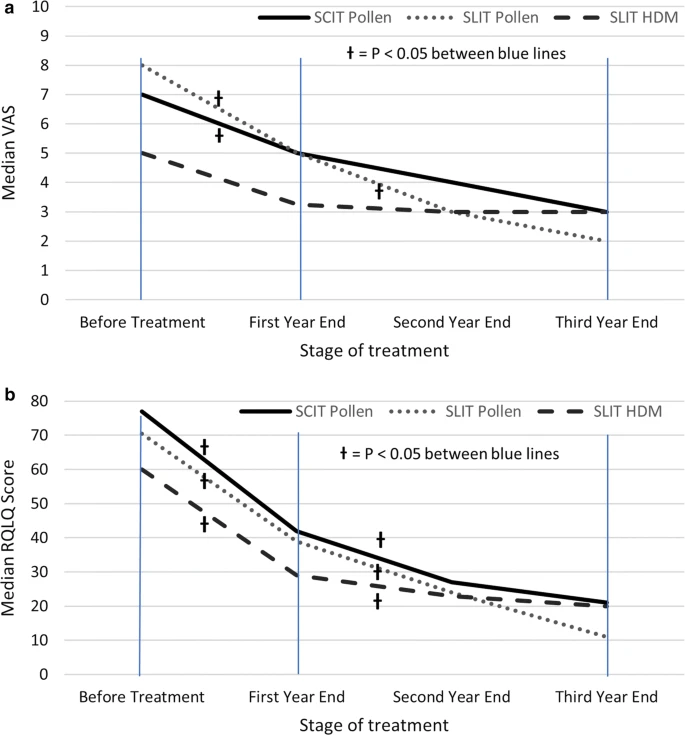
All three immunotherapy regimes led to a > 50% improvement in QoL and VAS after 3 years of therapy, with significant improvements by the end of the first year (p < 0.05) and further improvements between 1 and 3 years (p < 0.05). Age, gender, ethnicity and route of administration had no significant bearing on efficacy. Older, polysensitised children and those receiving HDM SLIT were all more likely to discontinue their treatment (all with p < 0.05). The only patient to suffer from anaphylaxis requiring intramuscular adrenaline, and 80% experiencing exacerbations of their asthma had received pollen SCIT.
Conclusions
Pollen SCIT and pollen and HDM SLIT all lead to significant improvements in QoL. The risk of anaphylaxis is low, but SCIT is associates with a 1 in 5 chance of asthma flares in the days after its administration. Discontinuation of therapy is more frequent in older, polysensitised children, and those undergoing HDM immunotherapy.
No comments:
Post a Comment