The Global Initiative for Asthma has only recently added tiotropium bromide as adjunct controller therapy in severe asthma (Step 4 or 5) in adults (2015) and children (2019). Although not yet approved for pediatric use by Health Canada, it has been occasionally offered by asthma specialists as a therapeutic trial in children with troublesome asthma or treatment for adverse effects. The objective of this study was to describe the indications and real-life clinical experience in initiating tiotropium in children with asthma.
We designed a retrospective mixed-method case series study of children aged 1–17 years who initiated tiotropium in our tertiary-care centre between 2013 and 2020. Clinical information was extracted from electronic medical records and tiotropium dispensing, from drug claims. Parents/children and physicians independently completed a questionnaire about treatment goals, perceived efficacy, safety, satisfaction, and lessons learned.
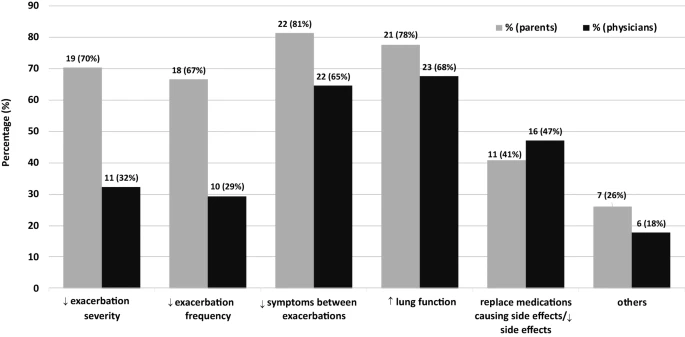
The 34 (11 females; 23 males) children had a median (range) age of 9.1 (1.4–17.8) years. Children were primarily on Step 4 (85%) or 5 (6%) prior to tiotropium initiation, yet most (84%) did not increase their treatment step after tiotropium initiation. The physicians’ treatment goals were to improve asthma control, alleviate adverse effects of current therapy, and/or improve lung function. The most improved symptoms were coughing/moist cough, difficulty breathing, whistling breath, and bronchial secretions/mucus. Although most parents and physicians reported a significant benefit with tiotropium bromide, physicians particularly remarked, as their “lesson learned’, on the improvement in chronic symptoms in asthmatic children, particularly those with prominent moist cough and in lung function, in those with seemingly none (or incompletely) reversible obstruction as well as the ability to decrease the ICS and/or LABA dose to lessen adverse effects. A few physicians raised caution on the risk of lower adherence with an additional inhaler.
Conclusion
In children with severe asthma on Step 4 or 5, tiotropium bromide was primarily used as substitute, rather than additional, adjunct therapy to improve asthma control, alleviate adverse effects, and/or to improve lung function. The latter two indications, combined with its perceived effectiveness in children with prominent moist cough, also suggest additional indications of tiotropium to be formally explored.
No comments:
Post a Comment