Sabaté-Brescó, M., Quan, P.L. & Goikoetxea, M.J. Curr Treat Options Allergy 10, 267–282 (2023).
Abstract
Purpose of review
To outline currently validated in vitro tests for the diagnosis of drug hypersensivity reactions (DHRs) and to provide useful strategies to optimise the utility of these tools.
Recent findings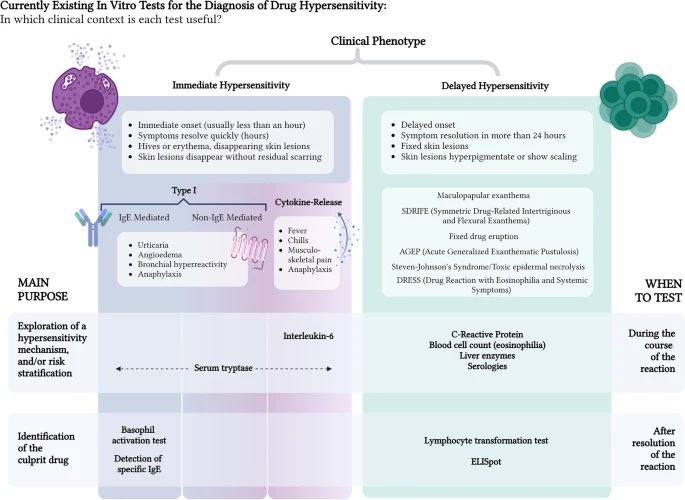
Most commonly used in vitro tests for the diagnosis of drug hypersensitivity.
Regarding in vitro tests for DHR, the main concern, at present, is low sensitivity. Thus, most of the efforts are currently directed towards improving the existing techniques and developing new assays with better diagnostic performance.
Summary
The management of DHRs is particularly challenging. Current strategies for diagnosis are focused on taking a thorough clinical history, evaluating sensitization using skin testing and performing supervised challenges. In vitro tests may potentially add information to the diagnostic algorithms for the management of DHRs.
The presently available assays, however, pose significant limitations in terms of availability and validation. Maximizing their yield and accuracy, therefore, requires a tailored approach, focused on an appropriate clinical characterisation of the reaction. The time elapsed between drug administration and symptom presentation, as well as symptom duration, should be closely taken into consideration. In this review, existing validated in vitro techniques that may support the diagnosis of both immediate and non-immediate DHRs are summarised. Clues for optimizing their diagnostic yield are given.
No comments:
Post a Comment