Article - Open access
Carl Skröder, Laila Hellkvist, Åslög Dahl, Ulla Westin, Leif Bjermer, Agneta Karlsson & Lars Olaf Cardell
Scientific Reports volume 13, Article number: 19649 (2023)Abstract
Intramuscular injections with methylprednisolone treating allergic rhinitis (AR) have a long history. Modern guidelines are designed to dissuade this treatment, but it´s frequently used, especially in primary care. This despite of concern for side effects and lack of modern placebo-controlled studies. This study was designed to evaluate if methylprednisolone, could significantly improve symptoms of birch pollen induced AR and reduce the concomitant use of standard of care medication. Forty-two patients with birch pollen induced AR were randomized to treatment with methylprednisolone (80 mg) or placebo (NaCl 0.9%). Daily symptom- and medication scores was registered for 3 weeks.
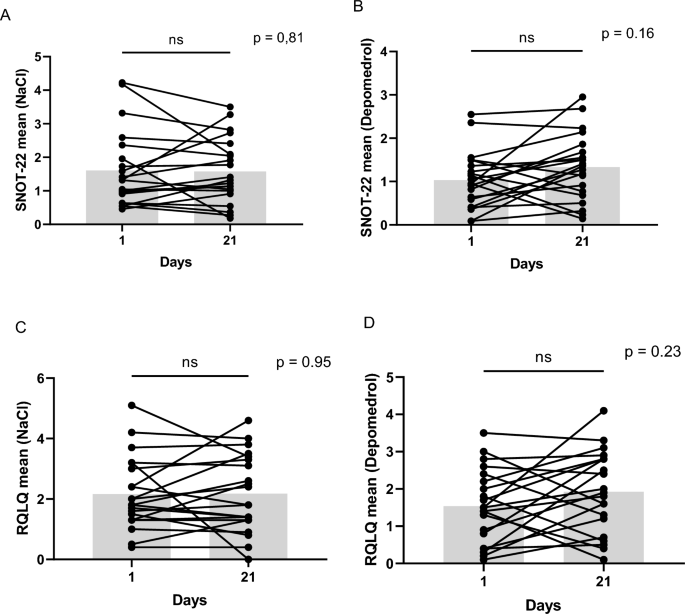 |
| (A,B) Individual changes in SNOT-22 in each group. (C,D) Individual changes in Juniper RQLQ in each group. |

No comments:
Post a Comment