Abstract
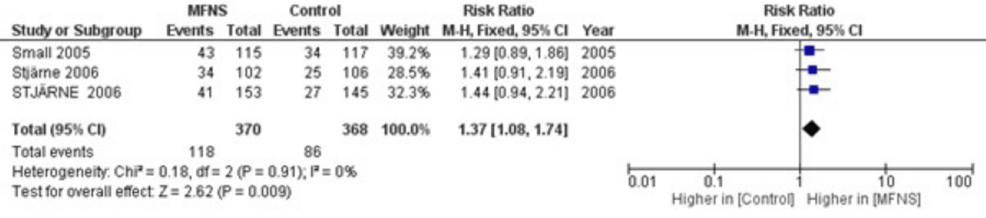 |
| Forest Plot of the Risk Ratio for Subjects With an
ImprovementWith MFNS Once Daily Compared to Placebo |
A blog that publishes updates and open access scientific papers about allergy, asthma and immunology. Editor: Juan Carlos Ivancevich, MD. Specialist in Allergy & Immunology
Abstract
 |
| Forest Plot of the Risk Ratio for Subjects With an
ImprovementWith MFNS Once Daily Compared to Placebo |
No comments:
Post a Comment