Epstein-Rigbi, N., Levy, M.B., Nachshon, L. et al. Allergy Asthma Clin Immunol 20, 53 (2024). https://doi.org/10.1186/s13223-024-00915-6
Abstract
Background
Oral immunotherapy (OIT) has become the standard of care for children with food allergy (FA) and has substantially improved their quality of life. The effect of OIT on the quality of life in adults, however, has been studied to a much lesser degree.
Methods
Patients with food allergy aged ≥ 18 years who underwent OIT at Shamir Medical Center completed the Food Allergy Quality of Life Questionnaire-Adult Form (FAQLQ-AF) before and at the end of treatment. Adults with FA not undergoing OIT who completed the FAQLQ-AF at 2 time points, served as controls.
Results
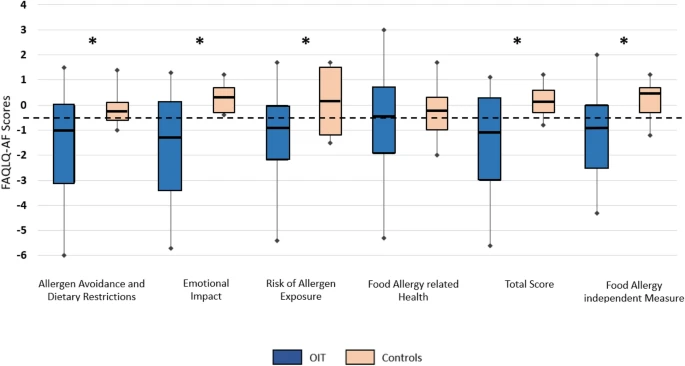 |
| Comparison of the delta in FAQLQ-AF scores between the study group (n = 44) and control group (n = 11). |
Conclusions
OIT significantly improves quality of life of adults with FA. This finding adds important support for providing OIT in this population.

No comments:
Post a Comment