Laikitmongkhon, J., Tassaneyasin, T., Sutherasan, Y. et al. BMC Pulm Med 24, 562 (2024). https://doi.org/10.1186/s12890-024-03364-4
Abstract
Background
The most appropriate anti-inflammatory treatment for moderate COVID-19 pneumonia remains uncertain. We aimed to compare the effectiveness of a high-dose methylprednisolone versus a high-dose dexamethasone in hospitalized moderate COVID-19 pneumonia, regarding the WHO clinical progression scales, mortality, and the length of hospitalization.
Methods
In this open-labeled randomized controlled trial, we enrolled patients with age > 18 years old who were diagnosed moderate COVID-19 pneumonia confirmed by real-time PCR, evidence of pneumonia by chest imaging and resting oxygen saturation between 90 and 94%. Patients were randomized at a 1:1 ratio to receive methylprednisolone 250 mg/day or dexamethasone 20 mg/day over the first three days.
Then the patients in both groups received dexamethasone 20 mg/day on days 4–5, and 10 mg/day on days 6–10. Primary outcome was assessed by a 10-point WHO clinical progression scales ranging from uninfected (point 0) to death (point 10) on the fifth day of treatment. Secondary outcomes including 90-day mortality, length of hospitalization, rate of intensive care unit (ICU) transfer and complications were determined.Results
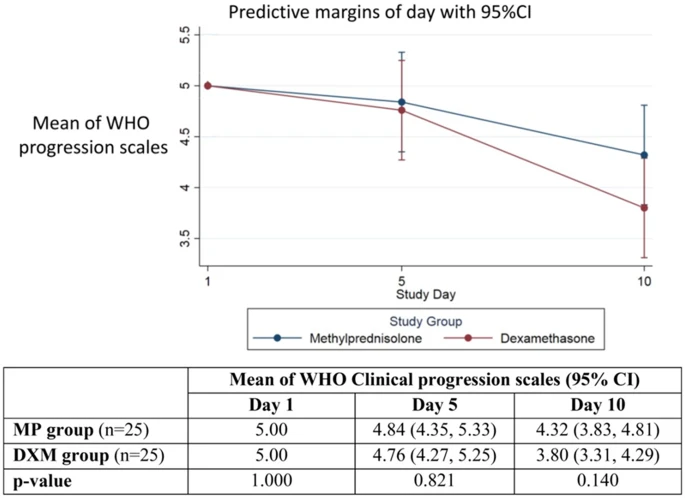 |
| Diagram of the mean (95% confidence interval) of WHO clinical progression scales at day 1, day 5, and day 10 in the MP group and DXM group and p-value bymultilevel mixed-effects linear regression. |
Conclusions
In patients with moderate COVID-19 pneumonia, initial anti-inflammatory treatment with 250 mg/day of methylprednisolone for three days does not yield better outcomes over high-dose dexamethasone.

No comments:
Post a Comment