Boursiquot, JN., Gagnon, R., Quirt, J. et al. Allergy Asthma Clin Immunol 20 (Suppl 3), 66 (2024). https://doi.org/10.1186/s13223-024-00935-2
Abstract
Allergen immunotherapy (AIT) is a potentially disease-modifying therapy that is effective for the treatment of allergic rhinitis/conjunctivitis, allergic asthma and stinging insect hypersensitivity. The decision to proceed with AIT should be made on a case-by-case basis, based on a comprehensive evaluation of the patient, allergy testing and a thorough discussion with the patient about treatment goals, risks vs. benefits, and long-term commitment to the treatment plan. For those with allergic rhinitis and/or asthma, it is also important to consider individual patient factors, such as the degree to which symptoms can be reduced by avoidance measures and pharmacological therapy, the amount and type of medication required to control symptoms, the adverse effects of pharmacological treatment, and patient preferences.
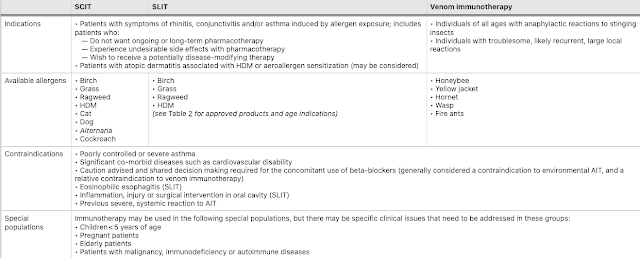 |
Indications, available allergens, contraindications and special considerations for AIT
Since AIT is associated with a risk of anaphylaxis, it should only be prescribed by physicians who are adequately trained in the treatment of allergic conditions.
Key take-home messages
AIT is a potentially disease-modifying therapy that is effective for the treatment of allergic rhinitis/conjunctivitis, allergic asthma and stinging insect hypersensitivity, as well as atopic dermatitis associated with HDM or aeroallergen sensitivity.
AIT is contraindicated in patients with uncontrolled or severe asthma, or those with significant co-morbid cardiovascular disease.
The use of beta-blockers is generally contraindicated for environmental AIT and is a relative contraindication for venom immunotherapy. Consideration of risk-benefit should be taken for concomitant use of ACE inhibitors.
The decision to proceed with AIT should be made on a case-by-case basis based on a thorough clinical evaluation, allergy testing and patient discussions regarding risks vs. benefits and long-term commitment to the treatment plan. In patients with allergic rhinitis and/or asthma, it is also important to take into account individual patient factors such as disease severity, efficacy of avoidance measures and pharmacological therapy, and patient preferences for disease-modifying therapy vs. conventional treatment.
AIT carries the risk of anaphylactic reactions and, therefore, should only be prescribed by physicians who are adequately trained in the treatment of allergy.
SCIT must be given under medical supervision in clinics that are equipped to manage life-threatening anaphylaxis.
SLIT is available in Canada for the treatment of HDM, grass, birch and ragweed allergy. It offers multiple potential benefits over SCIT including the comfort of avoiding injections, the convenience of home administration, and a favourable safety profile.

No comments:
Post a Comment