Abstract
Background
Immunoglobulin replacement therapy (IgRT) is the current standard of care for primary antibody deficiency patients (majority of all primary immunodeficiency (PID) diseases), with growing real-world evidence supporting use for secondary immunodeficiency (SID) patients. Infusion methods and practices can affect patients’ satisfaction with their treatment and perception of their health-related quality of life.
Methods
An online survey of US patients with PID and SID was conducted. This research investigates primarily the impact of two IgRT infusion methods, intravenous immunoglobulin therapy (IVIG) and subcutaneous immunoglobulin (SCIG), on the patient reported outcome (PRO) Life Quality Index (LQI) tool.
Patient reported infusion time efficiency, physical and mental health (PROMIS GPH-2 and PROMIS GMH-2 respectively), patient acceptability of their symptom state (PASS), upper extremity disability (Quick DASH) and general health perception (via the GHP) are also investigated.Results
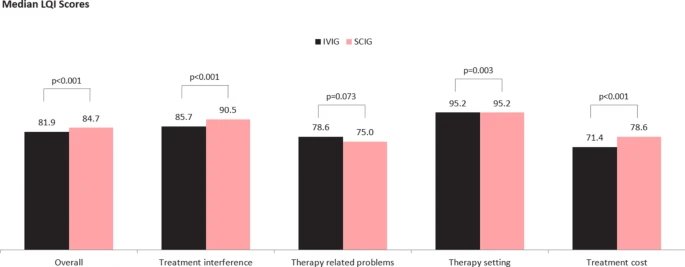 |
| Median LQI Scores |
Conclusions
The significantly higher LQI scores for patients receiving SCIG than those receiving IVIG confirms existing evidence that substitution of SCIG for IVIG may favorably impact immunoglobulin specific perceptions of quality of life and treatment satisfaction for appropriately selected patients. Our evidence on infusion times indicates similar improvement may be possible on infusion time efficiency.

No comments:
Post a Comment