Shamriz, O., Parnasa, E., Rubin, L. et al. BMC Immunol 26, 29 (2025). https://doi.org/10.1186/s12865-025-00705-8Abstract
Background
Physicians may encounter situations where they need to co-administer omalizumab with non–IgE-targeting monoclonal antibodies. In this study, we share our experience with these dual biologic treatments.
Objective
To evaluate the efficacy and safety of dual biological therapy using omalizumab and non-IgE-targeting monoclonal antibodies at a single center.
Methods
We retrospectively reviewed the medical records of adults treated with a dual biological therapy regimen consisting of omalizumab and another biologic between 2020 and 2022.
Results
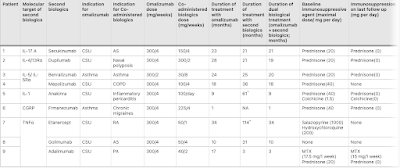
Our review identified nine patients (age range: 51–75 years, 7 women and 2 men) who were treated with omalizumab for high Th2 disorders, including chronic spontaneous urticaria (n = 7) and asthma (n = 2). Seven patients received a second biologic for co-existing non-Th2 disorders, while two received an additional biologic to better control their Th2-mediated disorders. The patients were treated with the following biologics: anti-IL-5 agents (mepolizumab [n = 1] and benralizumab [n = 1]), the IL-4/13 inhibitor dupilumab (n = 1), the anti-IL-17 biologic secukinumab (n = 1), the IL-1 inhibitor anakinra (n = 1), the anti-calcitonin gene–related peptide agent fremanezumab (n = 1), and anti-TNF-α agents (etanercept [n = 1], golimumab [n = 1], and adalimumab [n = 1]). Dual biotherapy was administered for 3–34 months with observed clinical improvement. No adverse events or infections were reported. Conclusions
Dual biological treatment with omalizumab and another biologic appears to be safe, with no need to discontinue non–IgE-targeting agents during omalizumab therapy.
PDF

No comments:
Post a Comment