TZ1391: a computationally designed circular mRNA multi-epitope vaccine candidate against Mycobacterium tuberculosis via TLR3 immunomodulation
Ali, A., Alamri, A., Mishra, V.K. et al. BMC Immunol (2026). https://doi.org/10.1186/s12865-025-00795-4
Abstract
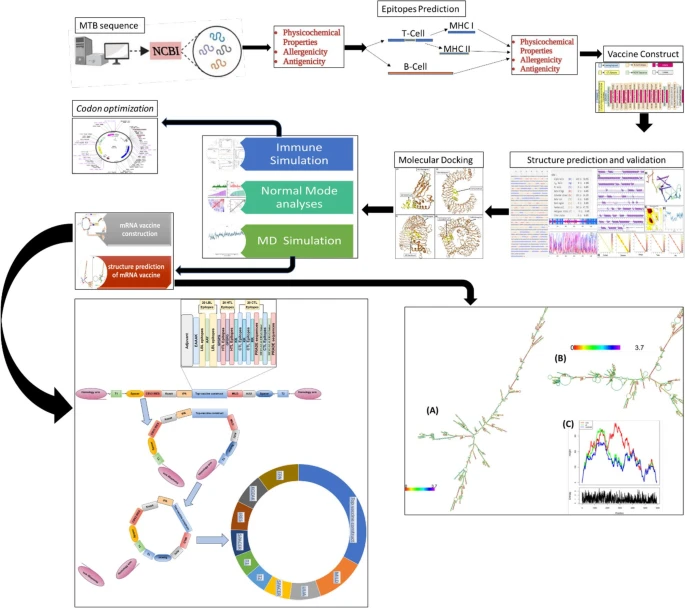 |
| Graphical Abstract |
Tuberculosis (TB), caused by Mycobacterium tuberculosis, remains a major global health burden due to latent infection, multidrug resistance, and the limited efficacy of the BCG vaccine. To address this challenge, we computationally designed and evaluated a circular mRNA-based multi-epitope vaccine candidate, TZ1391. Five experimentally validated M. tuberculosis antigens (ESAT-6, CFP-10, Ag85B, PPE18, and HspX) were used to predict immunodominant cytotoxic T lymphocyte (CTL), helper T lymphocyte (HTL), and B-cell epitopes. Three vaccine constructs (MTB-C1, MTB-C2, and MTB-C3) were assembled by integrating 20 CTL, 20 HTL, and 20 B-cell epitopes with appropriate linkers, PADRE sequence, and innate immune adjuvants. Structural modeling using AlphaFold2 and GalaxyRefine confirmed stable, native-like conformations for all constructs, with MTB-C3 showing the highest structural quality (GDT-HA = 0.8782; RMSD = 0.646 Å) and the greatest number of stabilizing disulfide bonds. Molecular docking against TLR3, TLR4, and TLR8 identified two top-performing candidates. MTB-C3 exhibited the strongest interaction with TLR3, achieving the lowest HDock score (− 480.53) and highest confidence score (0.9987), while MTB-C2 showed optimal binding to TLR4 (ClusPro score − 1488.6; confidence 0.9700). Despite favorable TLR4 engagement by MTB-C2, MTB-C3 was prioritized as the lead candidate (TZ1391) due to its superior structural stability, reduced conformational fluctuations during molecular dynamics simulations, and stronger TLR3 binding free energy (ΔG_bind = − 173.25 ± 7.9 kcal/mol). Immune simulations further predicted that TZ1391 elicits a robust Th1-biased response, characterized by sustained IgG production, strong IFN-γ and IL-2 induction, and durable immune memory. Overall, the strong TLR3-mediated interaction, combined with enhanced structural stability and favorable immunogenic profiles, establishes TZ1391 as a promising multi-epitope vaccine candidate for further experimental validation against tuberculosis.PDF


No comments:
Post a Comment