Original Investigation
Eric L. Simpson, MD1; Amy S. Paller, MS, MD2,3; Elaine C. Siegfried, MD4; et alMark Boguniewicz, MD5; Lawrence Sher, MD6; Melinda J. Gooderham, MD7,8,9; Lisa A. Beck, MD10; Emma Guttman-Yassky, MD, PhD11,12,13; David Pariser, MD14; Andrew Blauvelt, MD, MBA15; Jamie Weisman, MD16; Benjamin Lockshin, MD17,18; Thomas Hultsch, MD19; Qin Zhang, PhD20; Mohamed A. Kamal, PharmD, PhD20; John D. Davis, PhD20; Bolanle Akinlade, MD, MBA20; Heribert Staudinger, MD, PhD21; Jennifer D. Hamilton, PhD20; Neil M. H. Graham, MBBS, MD, MPH20; Gianluca Pirozzi, MD, PhD21; Abhijit Gadkari, PhD20; Laurent Eckert, PhD22; Neil Stahl, PhD20; George D. Yancopoulos, MD, PhD20; Marcella Ruddy, MD20; Ashish Bansal, MD20
JAMA Dermatol. Published online November 6, 2019. doi: https://doi.org/10.1001/jamadermatol.2019.3336
Key Points
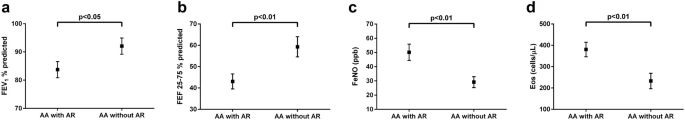
 Findings In this randomized phase 3 clinical trial including 251 adolescents with moderate to severe atopic dermatitis, dupilumab 200 or 300 mg every 2 weeks and 300 mg every 4 weeks resulted in a significant treatment response vs placebo following 16-week treatment, with an acceptable safety profile.
Findings In this randomized phase 3 clinical trial including 251 adolescents with moderate to severe atopic dermatitis, dupilumab 200 or 300 mg every 2 weeks and 300 mg every 4 weeks resulted in a significant treatment response vs placebo following 16-week treatment, with an acceptable safety profile.
Question What is the efficacy and safety of dupilumab monotherapy in adolescents with moderate to severe inadequately controlled atopic dermatitis?
 Findings In this randomized phase 3 clinical trial including 251 adolescents with moderate to severe atopic dermatitis, dupilumab 200 or 300 mg every 2 weeks and 300 mg every 4 weeks resulted in a significant treatment response vs placebo following 16-week treatment, with an acceptable safety profile.
Findings In this randomized phase 3 clinical trial including 251 adolescents with moderate to severe atopic dermatitis, dupilumab 200 or 300 mg every 2 weeks and 300 mg every 4 weeks resulted in a significant treatment response vs placebo following 16-week treatment, with an acceptable safety profile.



 Article Info
Article Info