- Letter to the Editor
- Open Access
Abstract
Background
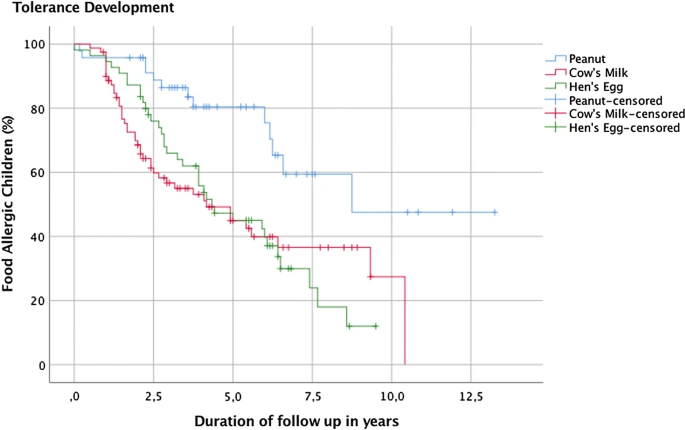 |
| Kaplan-Meier survival curve in peanut, cow’s milk and hen’s egg allergic children |
Tolerance development rates differ between food allergies. Almost all previous studies have not used the gold standard method, the double-blind, placebo-controlled food challenge (DBPCFC), which may affect the reported prevalence rates. Little is known about the association of the eliciting dose (ED) obtained during the initial DBPCFC with later tolerance development.


.png)