Abstract
 |
| Forest plot for clinical outcomes compared to other AR medication group. |
A blog that publishes updates and open access scientific papers about allergy, asthma and immunology. Editor: Juan Carlos Ivancevich, MD. Specialist in Allergy & Immunology
Abstract
 |
| Forest plot for clinical outcomes compared to other AR medication group. |
Louis G, Pétré B, Sousa-Pinto B, Bousquet J, Van Ganse É, Schleich F, Louis R. Sci Rep. 2024 Dec 2;14(1):29997. doi: 10.1038/s41598-024-81745-9.
While studies have demonstrated the impact of asthma symptoms on quality of life, very few studies have investigated the relationship between detailed asthma symptoms, as reported by the patient, and lung function and inflammation. A cross-sectional study was conducted on treated (ICS/LABA) adult (> 18 years) asthma patients recruited from the Liege University Hospital Asthma Clinic (Belgium) between 2018 and 2023 (n = 505).
Abstract
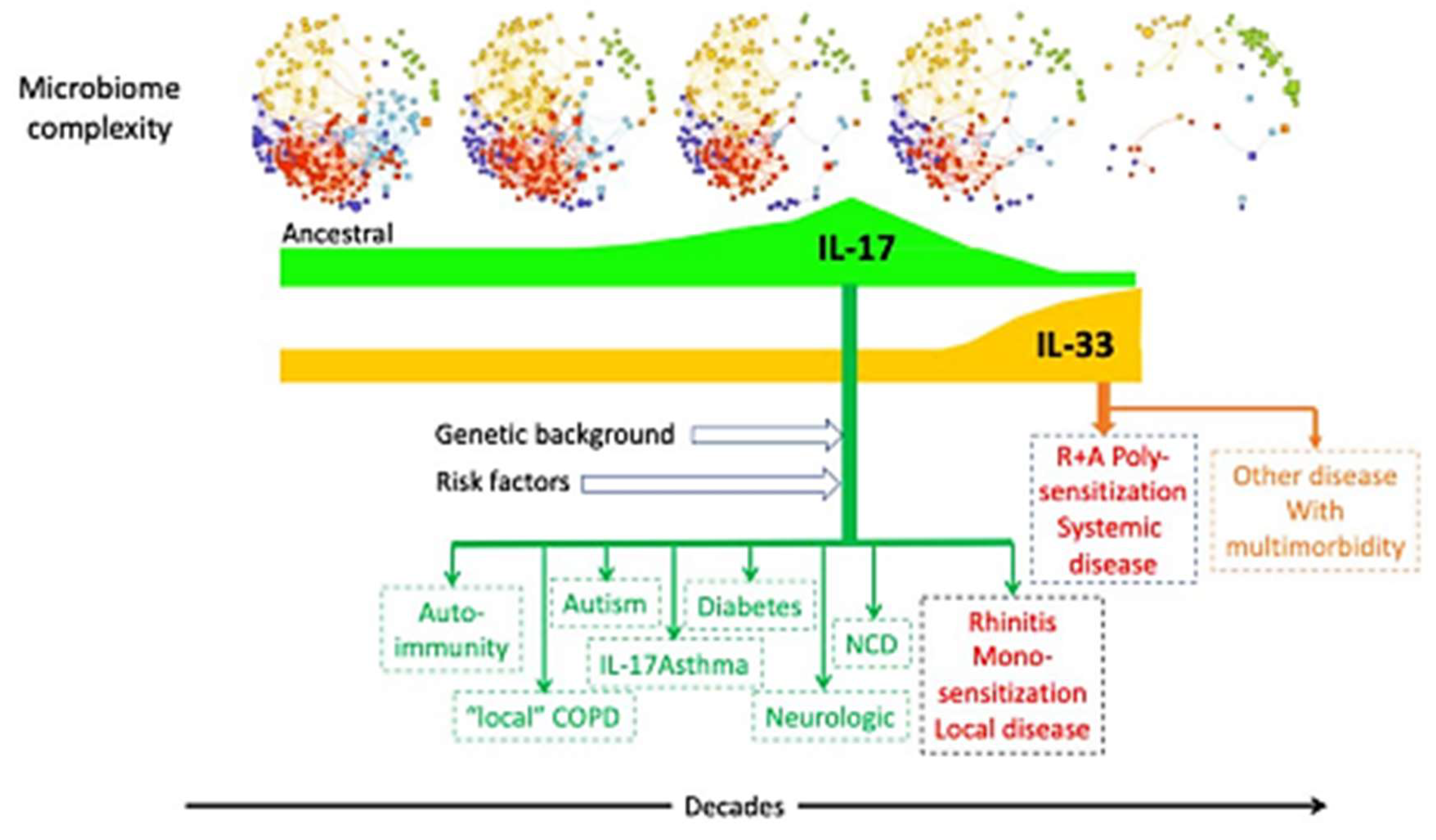 |
| Putative mechanisms between microbiome and diseases. |
Calvo, Silvia et al. eBioMedicine, Volume 107, 105272
Summary
Background
MTBVAC is a live attenuated tuberculosis vaccine, currently undergoing phase III evaluation for tuberculosis prevention. In previous preclinical studies, we found that local pulmonary administration of MTBVAC via the intranasal route had a strong therapeutic effect against asthma. This effect correlated with the abrogation of allergen-specific Th2 response in the lungs.
Methods
Using different mouse models of asthma, we investigated the effect of MTBVAC administered by intravenous (IV) route and its potential as immunotherapeutic agent to induce desensitisation of allergen-specific responses at a systemic level. We explored the effects of this process in the efficacy against airway hyperresponsiveness (AHR) induced by exposure to different allergens.
Findings
 |
| IV MTBVAC efficacy in two models of established asthma |
Abstract
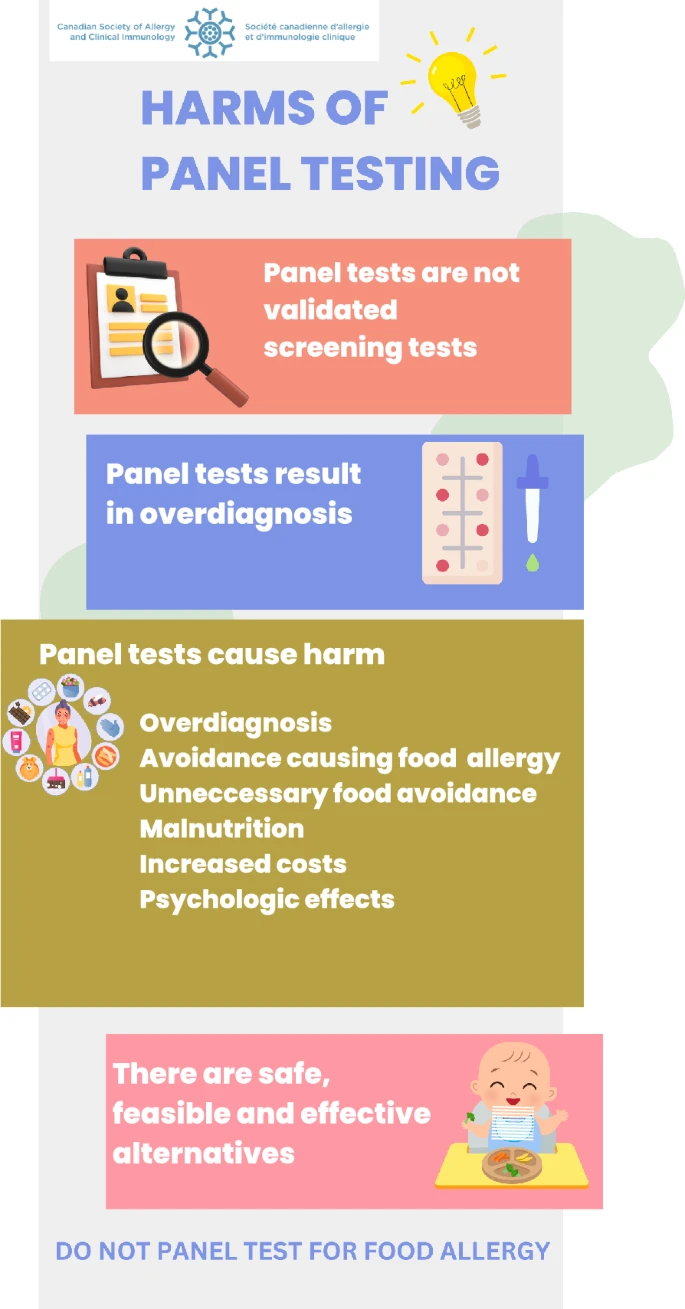 |
| Infographic for patient and clinician education regarding the harms of panel food testing |
Jutel M, Vogelberg C, Duwensee K, Troyke D, Klimek L. Allergy. 2024 Nov 14. doi: 10.1111/all.16370.
Abstract
Background
Allergen immunotherapy (AIT) aims at modulating the immune response by administration of allergen preparations at regular intervals over several years (1). For subcutaneous AIT (SCIT), the treatment is initiated with a dose escalation phase followed by a maintenance dose administration. Over the last decade, there has been a trend towards shortening dose escalation regimens to increase patient adherence. This open-label, phase II trial aimed to investigate the safety and tolerability of a house dust mites (HDMs) SCIT product when used in a newly designed one-strength dose escalation scheme.
Method
Patients, aged 12–65, suffering from HDM-allergic rhinitis/rhinoconjunctivitis ± asthma were included. Patients were randomized to the one-strength (6 injections from the highest strength 3) or the Standard dose escalation regimen (14 injections from strengths 1 to 3) using the HDMs-SCIT product. All adverse events were reported.
Ljung A, Gio-Batta M, Hesselmar B, Imberg H, Rabe H, Nowrouzian FL, Johansen S, Törnhage CJ, Lindhagen G, Ceder M, Lundell AC, Rudin A, Wold AE, Adlerberth I. PLoS One. 2024 Nov 27;19(11):e0313078. doi: 10.1371/journal.pone.0313078.
Abstract
Background
Children growing up on farms or with pets have a lower risk of developing allergy, which may be linked to their gut microbiota development during infancy.
Methods
Children from the FARMFLORA birth cohort (N = 65), of whom 28 (43%) lived on a dairy farm and 40 (62%) had pets, provided fecal samples at intervals from 3 days to 18 months of age. Gut microbiota composition was characterized using quantitative microbial culture of various typical anaerobic and facultatively anaerobic bacteria, with colonization rate and population counts of bacterial groups determined at the genus or species level.