A blog that publishes updates and open access scientific papers about allergy, asthma and immunology. Editor: Juan Carlos Ivancevich, MD. Specialist in Allergy & Immunology
May 10, 2025
Prenatal Ambient Air Pollution Associations With DNA Methylation in Asthma and Allergy Relevant Genes: Findings from ECHO
May 9, 2025
Rhinovirus as a driver of airway T cell dynamics in children with treatment-refractory recurrent wheeze
Bryant N, Muehling LM, Wavell K, Teague WG, Woodfolk JA. JCI Insight. 2025 May 8;10(9):e189480. doi: 10.1172/jci.insight.189480.
Abstract
 |
| Graphical Abstract |
Prevalence of Intolerance to Amines and Salicylates in Individuals with Atopic Dermatitis: A Systematic Review and Meta-Analysis
Fischer, K.; Jones, M.; O’Neill, H.M.Nutrients 2025, 17, 1628. https://doi.org/10.3390/nu17101628
Abstract
 |
| Graphical abstract |
Methods: Following the PRISMA guidelines, searches of PubMed, Embase, CINAHL, and Cochrane were conducted. Included studies involved children and adults with atopic dermatitis who underwent dietary elimination and double-blind placebo-controlled challenges involving histamine, other amines, or salicylates.
May 8, 2025
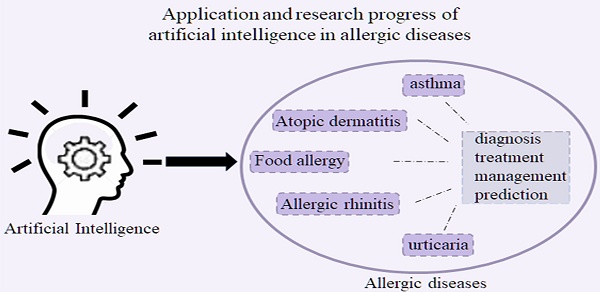
Application and research progress of artificial intelligence in allergic diseases
May 6, 2025
Rilzabrutinib in Antihistamine-Refractory Chronic Spontaneous Urticaria The RILECSU Phase 2 Randomized Clinical Trial
Giménez-Arnau A, Ferrucci S, Ben-Shoshan M, et al. JAMA Dermatol. Published online April 23, 2025. doi:10.1001/jamadermatol.2025.0733
Key Points
Findings In this randomized clinical trial of 160 patients with moderate to severe CSU, rilzabrutinib, 1200 mg/d, significantly decreased patients’ weekly Urticaria Activity Score and its components (weekly Itch Severity Score and weekly Hives Severity Score) at week 12 and as early as week 1. No new risks were observed.
Meaning Rilzabrutinib reduced itch and hives while maintaining a favorable risk-benefit profile, suggesting rilzabrutinib may be an efficacious treatment for patients with antihistamine-refractory moderate to severe CSU.
Importance Chronic spontaneous urticaria (CSU) is a skin disease driven mainly by the activation of cutaneous mast cells through various mechanisms.
May 4, 2025
Clinical Benefits of a Randomized Allergy App Intervention in Grass Pollen Sufferers: A Controlled Trial.
ABSTRACT
Background
Symptom monitoring can improve adherence to daily medication. However, controlled clinical trials on multi-modular allergy apps and their various functions have been difficult to implement.
 |
| Graphical Abstract |
Methods
We performed a stratified, controlled intervention study (May–August 2023) with grass pollen allergic participants (N = 167) in Augsburg, Germany.
Dermatologic presentations of hyper IgE syndrome in pediatric patients
Mahjoubi, M., Rashedi, R., Samieefar, N. et al. Allergy Asthma Clin Immunol 21, 20 (2025). https://doi.org/10.1186/s13223-025-00963-6
Abstract
Background
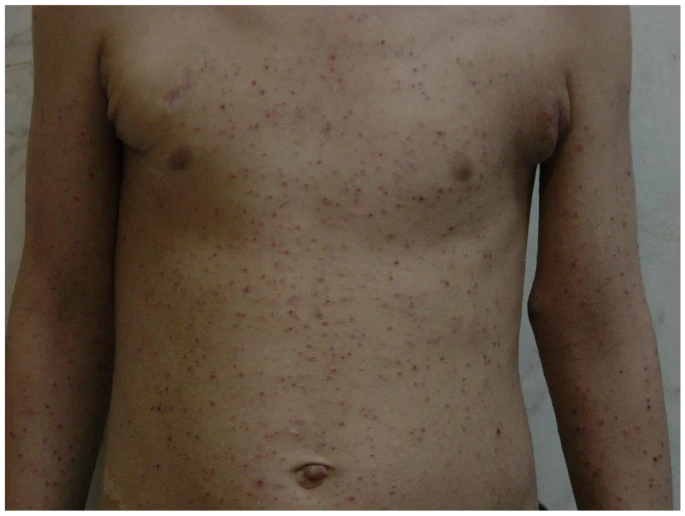 |
| Generalized erythematous and excoriated papules suggesting an eczematous dermatitis in a 10-year-old boy with HIES |
Methods
This narrative review explores the dermatologic presentations of Hyper-IgE Syndrome in pediatric populations
May 3, 2025
First-in-Class Intranasal Epinephrine Spray for Anaphylaxis: Dose Finding Clinical Study
Abstract
Background
Anaphylaxis is a life-threatening clinical presentation of acute systemic allergic reactions. Timely administration of epinephrine, usually by intramuscular autoinjector, is a robust life-saving treatment. Despite the critical necessity, there are multiple deterrants to patients’ proper use of epinephrine autoinjectors. FMXIN002 is a novel nasal dry powder formulation of epinephrine in a single-use device, offering first-in-class alternative treatment.
Objective
To measure epinephrine pharmacokinetics, pharmacodynamics and safety following a single administration of FMXIN002 at doses of 3.6 and 4.0 mg epinephrine versus IM autoinjector 0.3 mg, in healthy adults.
Methods
An open-label, single-dose, three-treatment, crossover, randomized, comparative bioavailability study with 12 healthy adults, female and male. FMXIN002 stability was also tested.
Results