Mishra T, Sasanka K, Sudha TY S, et al. (May 23, 2025) Cureus 17(5): e84705 doi:10.7759/cureus.84705
Abstract
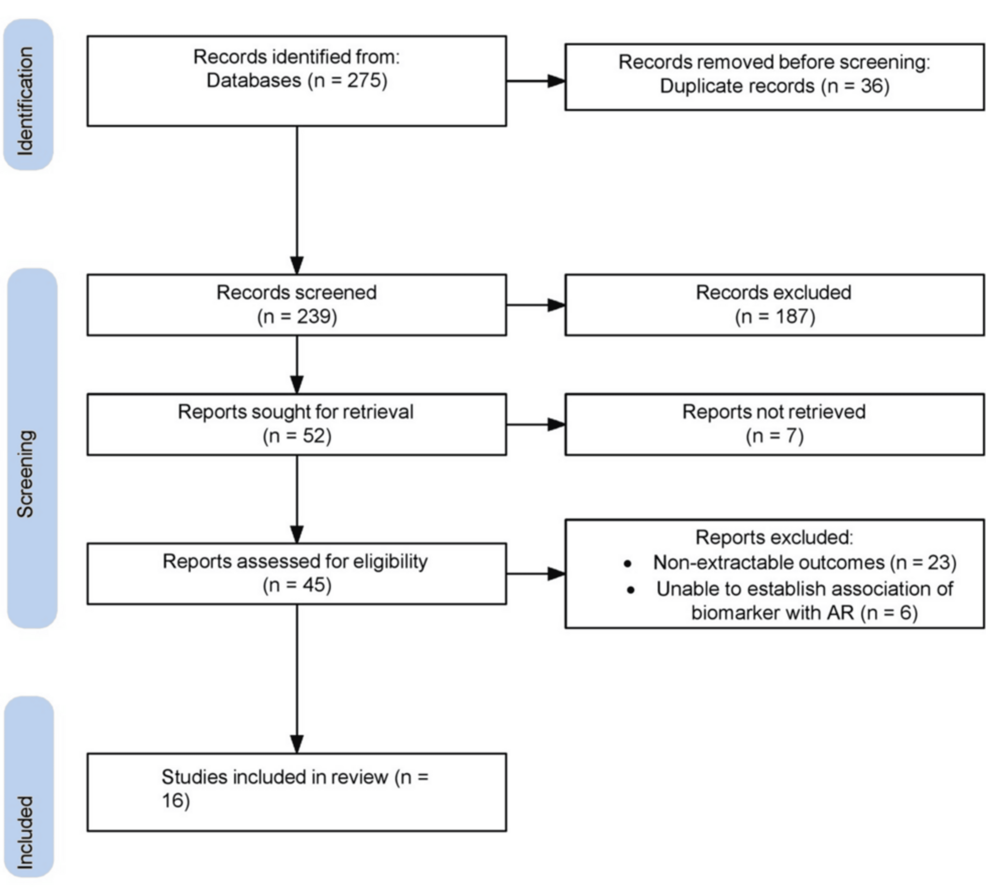 |
| Flow diagram depicting study search and screening |
A blog that publishes updates and open access scientific papers about allergy, asthma and immunology. Editor: Juan Carlos Ivancevich, MD. Specialist in Allergy & Immunology
Mishra T, Sasanka K, Sudha TY S, et al. (May 23, 2025) Cureus 17(5): e84705 doi:10.7759/cureus.84705
Abstract
 |
| Flow diagram depicting study search and screening |
Liu, L., Zhang, C., Xu, J. et al. BMC Pulm Med 25, 242 (2025). https://doi.org/10.1186/s12890-025-03485-4
To probe the diagnostic value of direct eosinophils (EOS) count and vascular endothelial growth factor (VEGF) in bronchial asthma (BA) and their correlation with inflammatory factors and lung function indicators.
A total of 66 patients with BA (BA group) were retrospectively gathered, who were further divided into mild (n = 25), moderate (n = 31), and severe (n = 10) subgroups based on asthma severity. Additionally, 60 healthy individuals undergoing physical examinations during the same period were enrolled as the normal group. The EOS count, serum VEGF, inflammatory factors [interleukin-6 (IL-6), interleukin-7 (IL-7), interleukin-10 (IL-10)], and lung function indicators [forced expiratory volume in one second (FEV1%) as a percentage of the predicted value, FEV1/forced vital capacity (FVC)] were compared among different groups.
Dahal, A., Chang, W.C., Johansson, E. et al. Sci Rep 15, 17606 (2025). https://doi.org/10.1038/s41598-025-99463-1
Staphylococcus aureus (SA) skin colonization in pediatric atopic dermatitis (AD) increases risk for severe AD and development of other allergic diseases. Despite this, there is no consensus regarding the optimal method to detect SA. Studies comparing metagenomic shotgun sequencing (MSS) and culture-based methods in SA detection and relationships to AD outcomes are lacking. In the Mechanisms of Progression of Atopic Dermatitis to Asthma in Children (MPAACH) cohort, we defined SA colonization categories by contact agar plate sampling/culture and skin tape sampling/MSS: double negative, sequencing only positive, contact plate only positive, and double positive (n = 759). 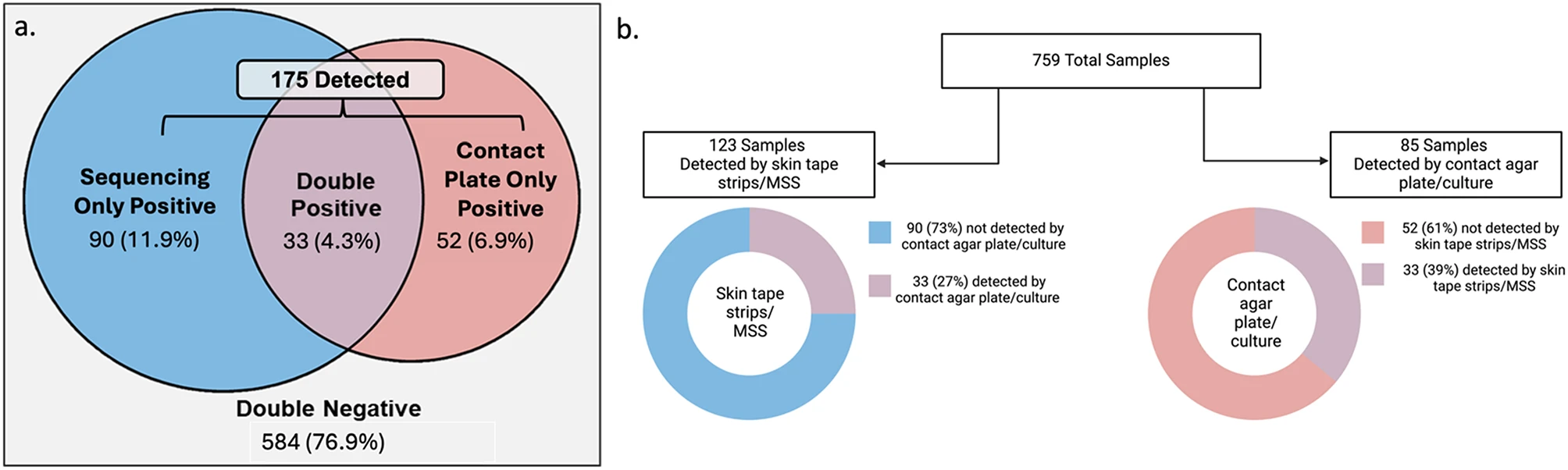
Total samples categories and detection rates by different methodologies
Key Points
Question Does incorporating chest computed tomographic imaging abnormalities and respiratory symptoms into the chronic obstructive pulmonary disease (COPD) diagnostic schema improve identification of individuals with poor respiratory outcomes?
Findings Among 9416 participants enrolled in a multicenter cohort study, those with newly diagnosed COPD had greater all-cause and respiratory-specific mortality, more frequent exacerbations, and faster decline of forced expiratory volume in the first second of expiration compared with individuals classified as not having COPD based on the new classification schema.
van der Gang LF, Atash K, Zuithoff NPA, Haeck I, Boesjes CM, Bacoş-Cosma OI, et al. J Eur Acad Dermatol Venereol. 2025; 00: 1–13. https://doi.org/10.1111/jdv.20674
Limited data exist on the comparative risk of infections during biologic and Janus kinase inhibitor (JAKi) treatment for atopic dermatitis (AD) in daily practice.
To assess the differential infection risk of biologic and JAKi treatment in patients with moderate-to-severe AD in a real-world setting.
This prospective, multicentre study evaluated treatment-emergent infections in patients (age ≥ 12 years) using biologics or JAKi from the BioDay registry from October 2017 to July 2024. Crude incidence rates were calculated per 100 patient-years (PY) per treatment. Cox regression for recurrent events, adjusted for potential confounders, was used to estimate hazard ratios (HR) for the rate of infections, with subgroup and sensitivity analyses in bio-/JAKi-naïve patients.
 |
| Graphical Abstract |
In total 1793 patients were included (4044.1 PY; 1886 biologic treatment episodes (TEs); 480 JAKi), with 794 infections. JAKi showed higher infection rates (58.4–65.5/100 PY) compared to biologics (13.6–22.0), especially for herpes infections (n = 195, 24.6%; JAKi 13.6–19.8 vs. biologicals 3.0–3.6). Cox regression indicated increased rates with JAKi (abrocitinib HR 4.1, 95% CI: 3.1–5.5; baricitinib HR 4.2, 95% CI: 2.9–6.2; upadacitinib HR 4.0, 95% CI: 3.2–5.0; all p < 0.0001) and a slight increase with tralokinumab (HR 1.4, 95% CI: 1.0–2.0, p = 0.039) compared to dupilumab.
Shen J, Zheng X, Yan M, Feng M, Ding C, Xie S, Xu H. J Inflamm Res. 2025;18:6191-6202
https://doi.org/10.2147/JIR.S519126
Purpose: Seasonal allergic rhinitis (SAR) is a prevalent inflammatory condition, yet its molecular mechanisms and reliable biomarkers remain incompletely understood. This study aimed to identify key inflammation-related proteins and pathways associated with SAR by investigating seasonal proteomic profile variations and their correlations with SAR symptoms.Graphical Abstract
Patients and Methods: Serum samples were collected from nineteen SAR patients during both allergy (in-season, IS) and non-allergy (out-of-season, OS) periods. Differentially expressed proteins (DEPs) were identified using the Olink Target 96 Inflammation panel, which were further analyzed through Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses.