 |
| AI Integration Pathways in Primary Immunodeficiencies. Radial diagram summarizing the meeting discussion themes |
A blog that publishes updates and open access scientific papers about allergy, asthma and immunology. Editor: Juan Carlos Ivancevich, MD. Specialist in Allergy & Immunology
September 17, 2025
Proceedings of the Second Artificial Intelligence in Primary Immunodeficiencies (AIPI) Meeting
Stapokibart for Severe Uncontrolled Chronic Rhinosinusitis With Nasal Polyps The CROWNS-2 Randomized Clinical Trial
Shen S, Yan B, Wang M, et al. JAMA. 2025;334(11):962–972. doi:10.1001/jama.2025.12515
Key Points
Question Among patients with severe chronic rhinosinusitis with nasal polyps taking a daily intranasal corticosteroid, does stapokibart, a novel anti–interleukin 4Rα monoclonal antibody, decrease nasal polyps and reduce symptoms of nasal congestion?
Findings In this randomized clinical trial that included 179 participants from China, stapokibart significantly reduced polyp size and improved nasal symptoms at 24 weeks in patients with severe chronic rhinosinusitis with nasal polyps.
Meaning Among patients with severe chronic rhinosinusitis with nasal polyps treated with a daily intranasal corticosteroid, stapokibart reduced polyp size and severity of nasal symptoms at 24 weeks.
September 16, 2025
Validation of the Urticaria Activity Score for Cold Urticaria
Background
Cold urticaria (ColdU) is characterized by the appearance of wheals and/or angioedema and itch after exposure to cold stimuli. The Cold Urticaria Activity score (ColdUAS) is a newly devised patient-reported outcome measure (PROM) assessing disease activity in ColdU.
Objectives
We aimed to validate the ColdUAS according to PROM guidelines, assess the optimal documentation period, and develop a scoring computation algorithm.
Methods
We instructed 71 patients with typical and atypical ColdU to complete the ColdUAS questionnaire over 4 consecutive weeks and asked to fill out additional anchor instruments including global assessment tools and validated quality of life measures.
September 15, 2025
Efficacy and safety of Sublingual immunotherapy for allergic rhinitis: an overview of systematic reviews and meta analyses
Wang, Z., Wang, N., Liang, X. et al. Eur Arch Otorhinolaryngol (2025). https://doi.org/10.1007/s00405-025-09664-7
Abstract
Introduction
Reappraisal of Systematic reviews/Meta analyses on Sublingual Immunotherapy for Allergic Rhinitis: Evidence for Clinical Practice and Decision-Making.
Methods
A comprehensive computerized search was conducted across PubMed, Embase, the Cochrane Library, Web of Science, CNKI, VIP, WANFANG, and CBM databases from their inception to June 8, 2025, to systematically identify Systematic reviews and Meta analyses on Sublingual Immunotherapy for allergic rhinitis. A citation overlap matrix was constructed to calculate the corrected covered area, assessing the degree of primary study overlap. The risk of bias, methodological quality, reporting quality, and certainty of evidence in the included Systematic reviews/Meta analyses were evaluated using ROBIS, AMSTAR-2, PRISMA 2020, and GRADE tools, respectively. Both quantitative and qualitative analyses were performed on the primary outcomes to derive a more comprehensive and in-depth understanding.
Results
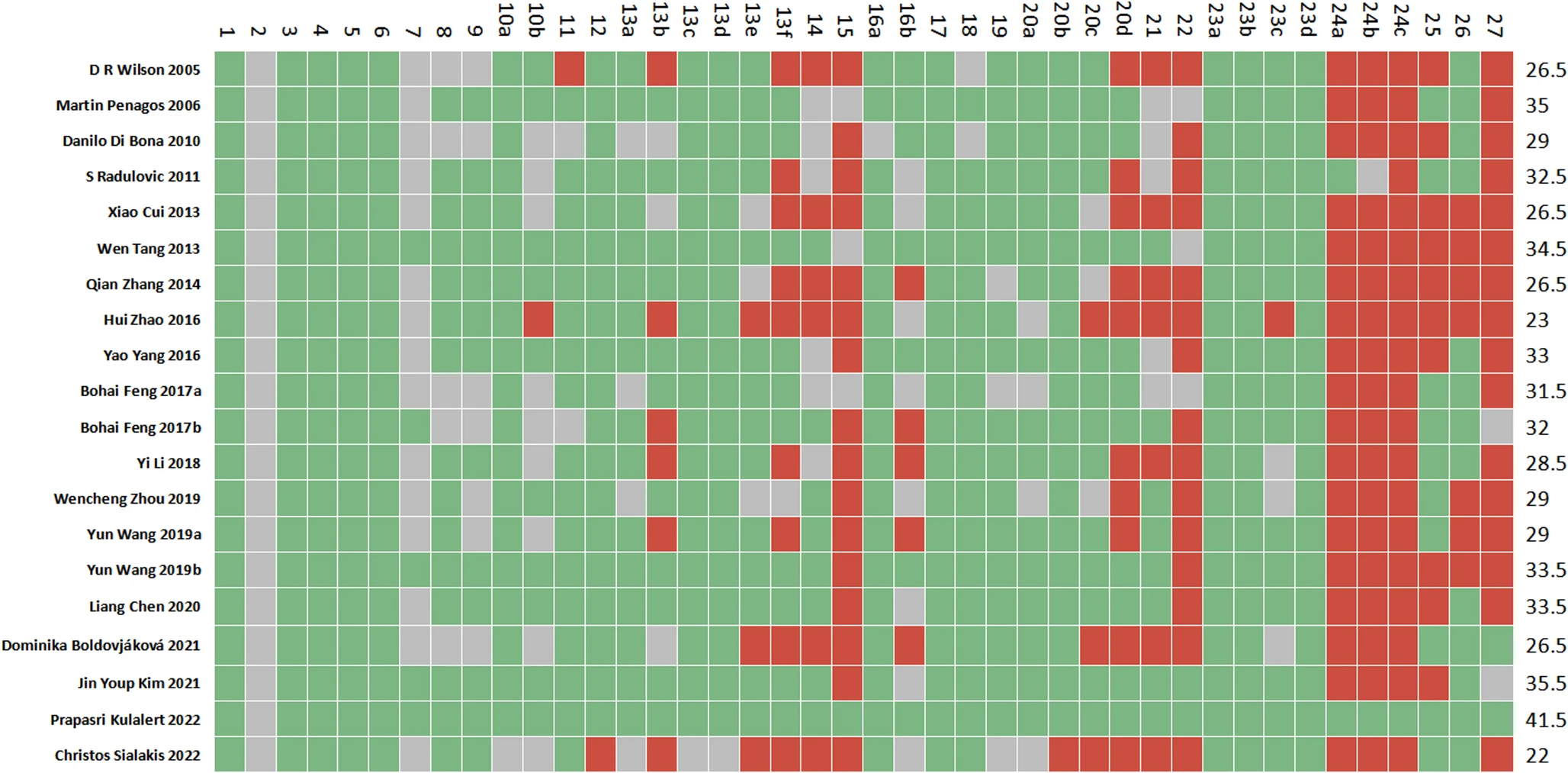 |
| Cartesian heatmap of the scores of each item in PRISMA 2020. Note: Green indicates compliance; Gray indicates partial compliance; Red indicates non-compliance |
Differential Diagnosis of Chronic Spontaneous Urticaria
Abstract
Patients with chronic recurrent wheals most commonly receive the diagnosis of chronic spontaneous urticaria, although a number of autoimmune, autoinflammatory, and malignant diseases can be suspected based on certain red flags. These warning signs are a wheal duration of more than 24 hours, post-inflammatory hyperpigmentation, and systemic symptoms such as arthralgia and fever and/or elevated inflammatory markers.
 |
| Urticarial exanthema: (A) Chest, (B) back, and (C) hands. |
September 11, 2025
Bibliometric analysis of the association between air pollution and allergic rhinitis
Geng Z, Ma Y, Qi X. Glob Health Action. 2025 Dec;18(1):2547434. doi: 10.1080/16549716.2025.2547434.
Abstract
Background: Allergic rhinitis (AR) is an increasingly prominent global public health issue, where air pollution significantly contributes to its rising incidence. Although numerous studies have explored the link between air pollution and AR pathogenesis, comprehensive summaries are still limited.
Objective: This study performs a bibliometric analysis to identify research hotspots and emerging trends, offering insights into AR prevention and management.
Methods: Literature related to on air pollution and AR was retrieved from the Web of Science Core Collection database. Visualization tools, including VOSviewer, CiteSpace, and Bibliometrix R, were utilized to analyze contributions by countries, institutions, authors, journals, and keywords, with the aim of predicting future research trends.
 |
| Number of global publications related to air pollution and allergic rhinitis |
Multiomics approach to evaluating personalized biomarkers of allergen immunotherapy
Shamji MH, Fulton WT, Animashaun I et al. J Allergy Clin Immunol. 2025 Sep;156(3):523-534. doi: 10.1016/j.jaci.2025.06.036.
Abstract
 |
| Multiomics approach to identify biomarkers of AIT. |
Comparative Safety of JAK Inhibitors vs TNF Antagonists in Immune-Mediated Inflammatory Diseases A Systematic Review and Meta-Analysis
Solitano V, Ahuja D, Lee HH, et al. JAMA Netw Open. 2025;8(9):e2531204. doi:10.1001/jamanetworkopen.2025.31204
Key Points
Question What are the comparative safety profiles of Janus kinase (JAK) inhibitors vs tumor necrosis factor (TNF) antagonists in patients with immune-mediated inflammatory diseases (IMIDs)?
Findings In this systematic review and meta-analysis including 42 head-to-head comparative effectiveness studies of 813 881 patients with IMIDs treated with JAK inhibitors or TNF antagonists, no meaningful differences in risk of serious infections, malignant neoplasms, and major cardiovascular events were observed. JAK inhibitor use was associated with a slightly higher risk of venous thromboembolism compared with TNF antagonist use; the overall incidence of serious adverse events was low.
Meaning These findings call for revisiting the strict regulatory guidance imposed by the US Food and Drug Administration and the European Medicines Agency restricting use of all JAK inhibitors after failure of, or contraindications to, TNF antagonists, across all indications.
Importance Janus kinase (JAK) inhibitors are highly effective medications for several immune-mediated inflammatory diseases (IMIDs). However, safety concerns have led to regulatory restrictions.





