C. Bachert, H. Hoogeveen, R. ten Have, et al. Allergy (2025): 1–10, https://doi.org/10.1111/all.70063.
ABSTRACT
Background
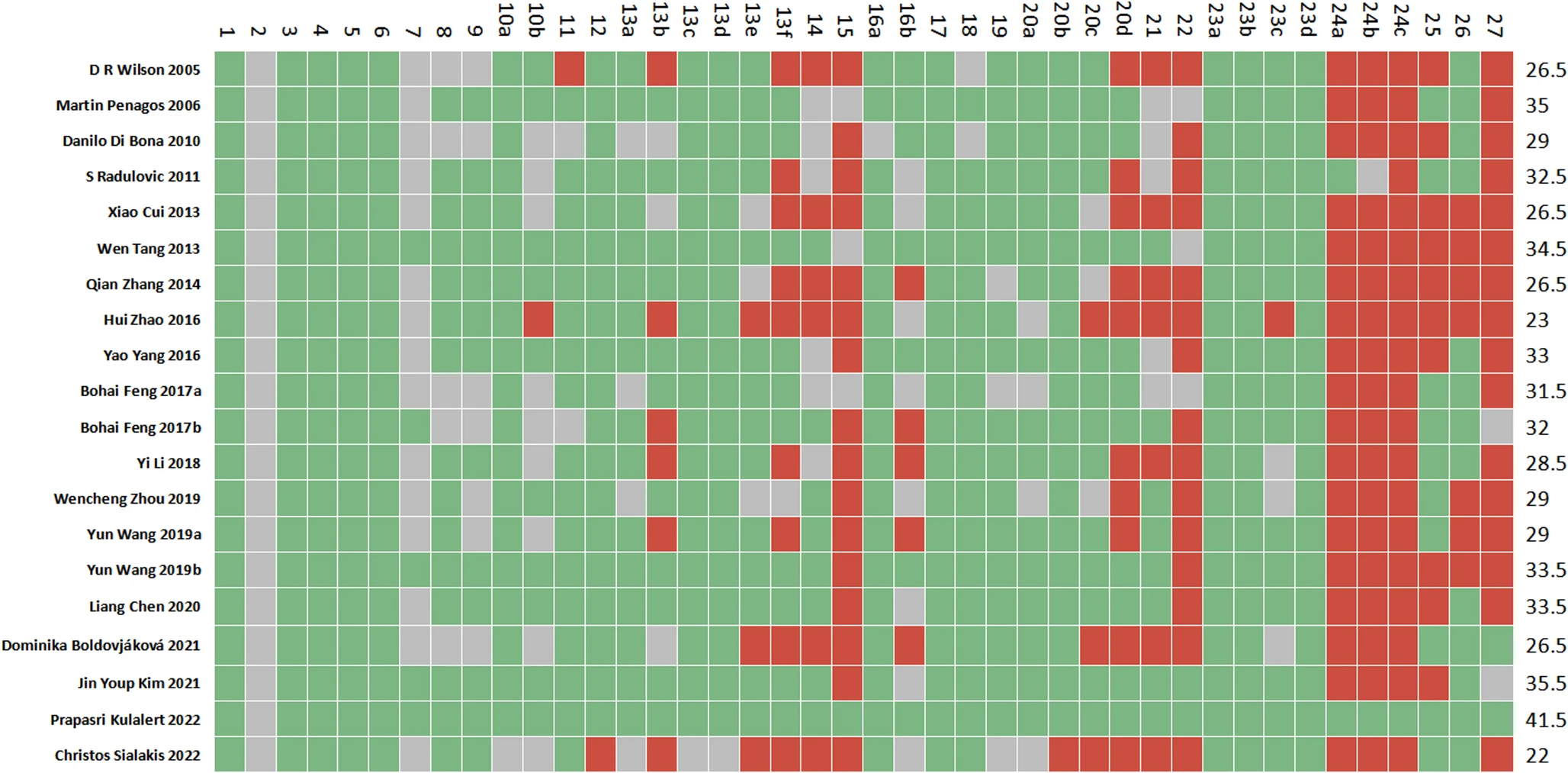
Subcutaneous allergen immunotherapy (SCIT) has a longstanding history as a safe and effective treatment. Nevertheless, to meet the European Medicines Agency's regulatory requirements for continued market authorization, its efficacy and safety must be confirmed in a pivotal Phase III trial.
 |
| Graphical Abstract |
Based on a successful Phase II dose-finding study, the aim was to confirm the safety and efficacy of a subcutaneous house dust mites (HDM) preparation in a randomized controlled trial using an optimal higher dose than the current maintenance dose.
Method
Seven hundred sixty-seven subjects were randomized in a 1:1 ratio to 1-year treatment with SCIT-product at a dose of 50,000 AUeq/mL or placebo.