Open Access
Letter to the Editor
In Europe, allergen products from different manufacturers can be labeled using the same unit with yet different definitions of that unit, which may cause confusion, as is the case for the index of reactivity (IR). In this context, house dust mite (HDM) Staloral 300 IR/mL, from Stallergenes Greer, and HDM Osiris 300 IR/mL, from ALK-Abelló, were characterized in vitro.
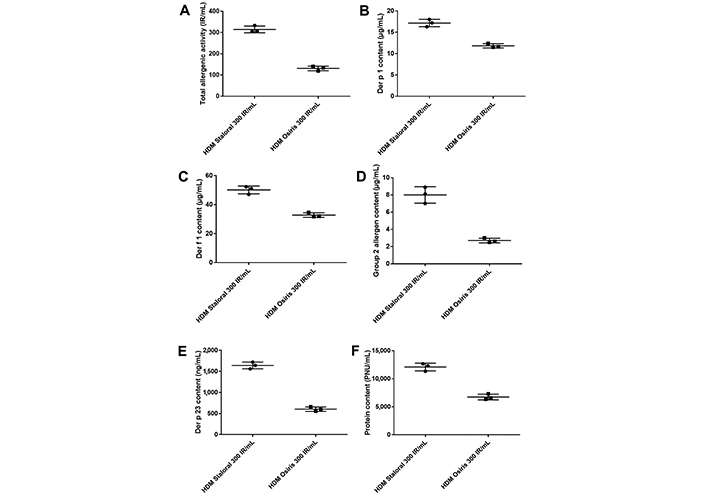
Qualitatively, namely in terms of protein and allergen profiles, the two products were similar. Quantitatively, and despite the same 300 IR/mL labeling, the two products were shown to have different biological potencies, with HDM Staloral 300 IR/mL displaying a 2.4 times higher total allergenic activity (TAA) than HDM Osiris 300 IR/mL. This higher biological potency of HDM Staloral 300 IR/mL was paralleled by higher allergen and protein contents, namely 1.5 times more Der p 1 and Der f 1, 3.0 times more group 2 allergens, 2.7 times more Der p 23, and 1.8 times more protein. In contrast, HDM Staloral 300 IR/mL was shown to contain far fewer culture medium-derived proteins than HDM Osiris 300 IR/mL.
No comments:
Post a Comment