Padilla-Galo, A., Moya Carmona, I., Ausín, P. et al. Respir Res 24, 235 (2023). https://doi.org/10.1186/s12931-023-02539-7
Abstract
Background
The ORBE II study aimed to describe the characteristics and clinical outcomes of adult patients with severe eosinophilic asthma (SEA) treated with benralizumab in a real-world setting in Spain.
Methods
ORBE II (NCT04648839) was an observational, retrospective cohort study in adult SEA patients who had been prescribed benralizumab. Demographic and clinical data of 204 SEA patients were collected 12 months prior to benralizumab initiation (baseline) and at follow-up. Exacerbation rate, asthma symptoms, maintenance oral corticosteroid (OCS) use and lung function were evaluated, among other variables.
Results
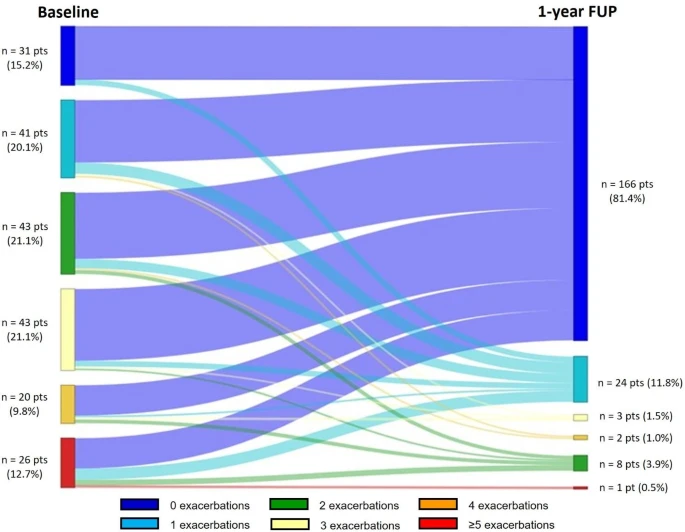 |
| Change in the number of exacerbations over 1-year follow-up. |
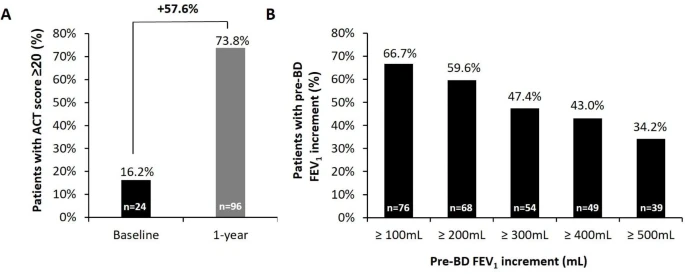 |
| Improvements in asthma control and lung function in the OP. |
After 1 year of follow-up, mean reduction in the daily OCS dose was 70.5%, and complete OCS withdrawal was achieved by 52.8% of the OCS-dependent patients. Almost half (43.7%) of the OP on benralizumab met all four criteria for clinical remission. Patients with concomitant CRSwNP obtained similar or enhanced outcomes.
Conclusions
These data support the real-world benefits of benralizumab in SEA patients, and particularly in those with concomitant CRSwNP.
No comments:
Post a Comment