Bernstein JA, Llanos JP, Hunter G, Martin N, Ambrose CS. Adv Ther. 2023 Sep 12. doi: 10.1007/s12325-023-02647-2.
Abstract
Patients with uncontrolled, allergic severe asthma may be prescribed biologic therapies to reduce exacerbations and improve disease control. Randomized controlled trials (RCTs) of these therapies have differed in design, with varying results overall and by baseline blood eosinophil count (BEC). This study describes published annualized asthma exacerbation rate (AAER) reductions from RCTs in patients with allergic severe asthma, overall and by baseline BEC category. A literature search was performed to identify published phase 3 RCT data of US Food and Drug Administration-approved biologics for severe asthma in patients with severe, uncontrolled asthma and confirmed sensitization to perennial aeroallergens.
Analyses focused on AAER reduction versus placebo in the overall population and/or in those with an elevated or low BEC at baseline or screening. Baseline serum total immunoglobulin E levels varied between RCT populations.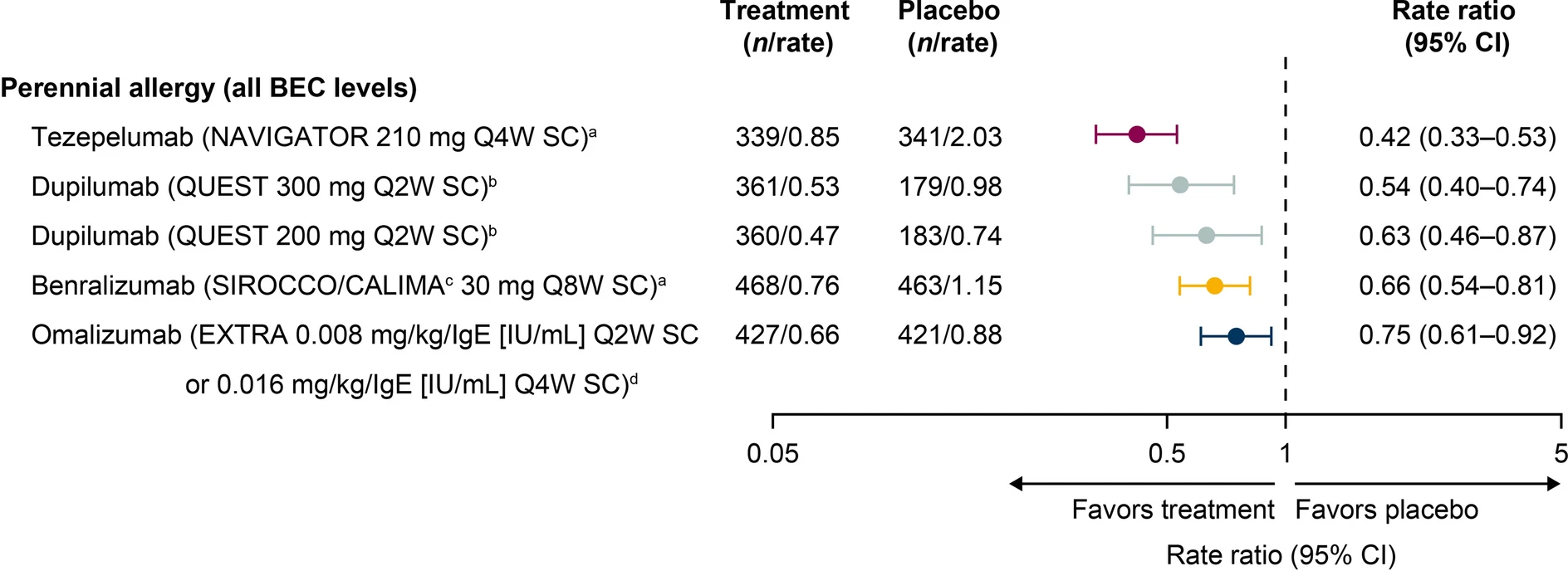 |
| Reduction in AAER by biologic therapy in patients with allergic severe asthma (all baseline BEC levels) |
Key Summary Points
- Randomized controlled trials (RCTs) of biologics in patients with severe asthma have demonstrated that efficacy varies in the overall allergic population and according to baseline blood eosinophil count (BEC).
- In the absence of head-to-head trials evaluating efficacy in patients with allergic severe asthma to date, a literature review was conducted to describe the effects of biologics on the annualized asthma exacerbation rate (AAER) in the overall allergic severe asthma population and by elevated and low baseline BEC in placebo-controlled, phase 3 RCTs.
- Among patients with allergic severe asthma, all biologics demonstrated efficacy in reducing the AAER in the overall population; greater reductions in the AAER were observed with higher baseline BEC.
- Efficacy in reducing the AAER varied between individual biologics, likely due to their differing mechanisms of action, and differences in study design, study populations and inclusion/exclusion criteria between trials.
- The findings in this study can help clinicians to compare efficacy data as well as inform provider treatment decisions when selecting biologic treatments for patients with allergic severe asthma overall and for those with or without coexisting eosinophilic inflammation.

No comments:
Post a Comment