Li, W., Tang, SC. & Jin, L. BMC Pulm Med 24, 70 (2024). https://doi.org/10.1186/s12890-024-02885-2
Abstract
Background
We aimed to clarify comprehensively the safety profiles of anti-IL-5 drugs and pinpoint potential safety concerns that may arise in their post-marketing phase.
Methods
Two researchers conducted comprehensive searches of PubMed, EMBASE, Web of Science, and the Cochrane Library from inception to September 2022. Additionally, we investigated the FDA AE Reporting System for post-marketing adverse event (AE) reports related to anti-IL-5 drugs.
The outcomes fulfilled the proportional reporting rate criteria and the Bayesian confidence propagation neural network.Results
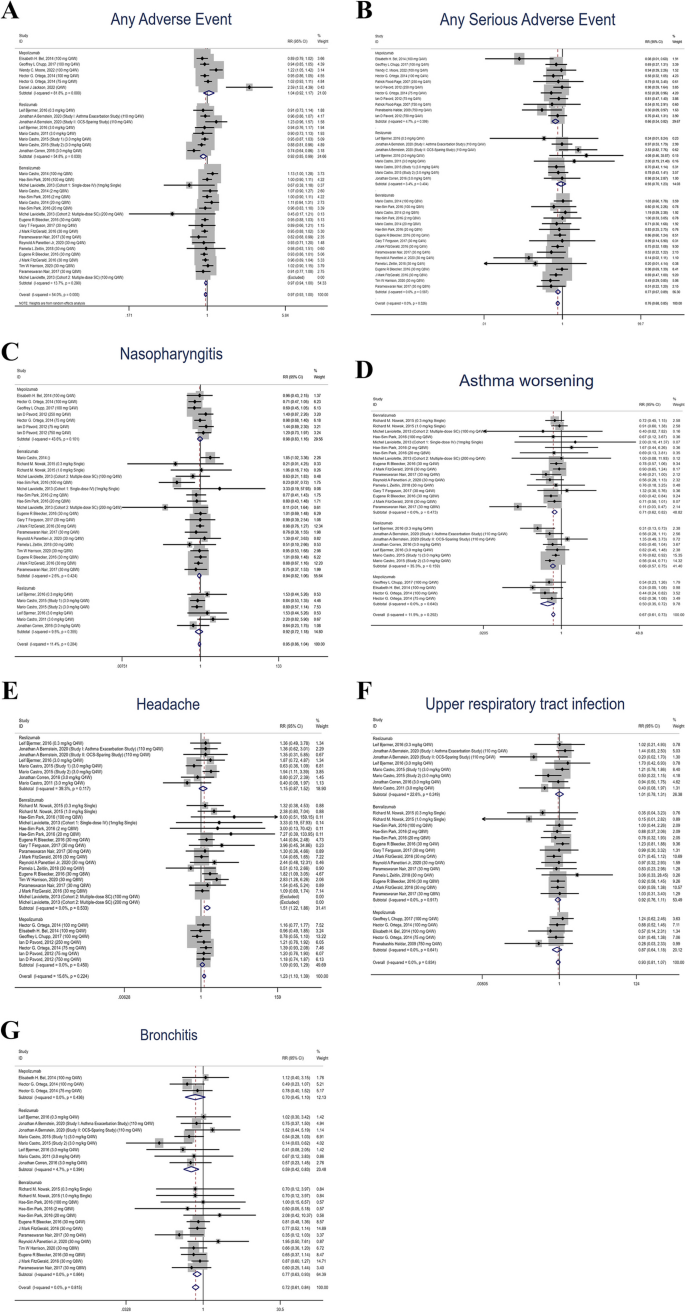
We included 24 published studies in our analysis. The anti-IL-5 treatment group showed an incidence of AEs comparable to the placebo group, and it exhibited a significantly lower frequency of serious AEs. Common AEs were asthma, nasopharyngitis, headache, upper respiratory tract infection (URTI), and bronchitis. The post-marketing data included 28,478 case reports associated with the suspect drugs and 75 suspect safety observations affecting 16 system organ classes. New suspect observations included incomplete therapeutic product effect, URTIs, and pulmonary mass in reports related to mepolizumab. Reports associated with mepolizumab and benralizumab also indicated issues with incorrect technique in device usage and product issues.
Conclusions
Individual anti-IL-5 drugs’ safety profiles largely matched their product inserts. We identified issues like improper device usage, product issue, and URTIs as potential concerns for mepolizumab and benralizumab. Additionally, all anti-IL-5 drugs showed signs of incomplete therapeutic effects.

No comments:
Post a Comment