Research - Open access
Bergwik, J., Liu, J., Padra, M. et al. Respir Res 25, 146 (2024). https://doi.org/10.1186/s12931-024-02780-8
Abstract
Background
In chronic pulmonary diseases characterized by inflammation and airway obstruction, such as asthma and COPD, there are unmet needs for improved treatment. Quinolines is a group of small heterocyclic compounds that have a broad range of pharmacological properties. Here, we investigated the airway relaxant and anti-inflammatory properties of a novel quinoline (RCD405).
Methods
The airway relaxant effect of RCD405 was examined in isolated airways from humans, dogs, rats and mice. Murine models of ovalbumin (OVA)-induced allergic asthma and LPS-induced airway inflammation were used to study the effects in vivo. RCD405 (10 mg/kg) or, for comparisons in selected studies, budesonide (3 mg/kg), were administered intratracheally 1 h prior to each challenge. Airway responsiveness was determined using methacholine provocation. Immune cell recruitment to bronchi was measured using flow cytometry and histological analyses were applied to investigate cell influx and goblet cell hyperplasia of the airways. Furthermore, production of cytokines and chemokines was measured using a multiplex immunoassay. The expression levels of asthma-related genes in murine lung tissue were determined by PCR. The involvement of NF-κB and metabolic activity was measured in the human monocytic cell line THP-1.
Results
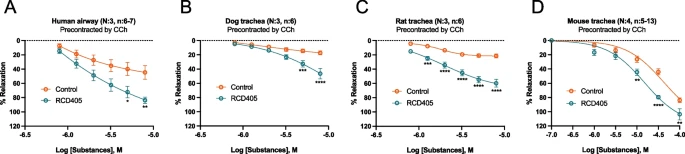 |
| The relaxing effect of RCD405 on human, dog, rat and mouse isolated airway pre-contracted with CCh. |