Seremet, T., Di Domizio, J., Girardin, A. et al. Nat Commun 15, 10688 (2024). https://doi.org/10.1038/s41467-024-54559-6
Abstract
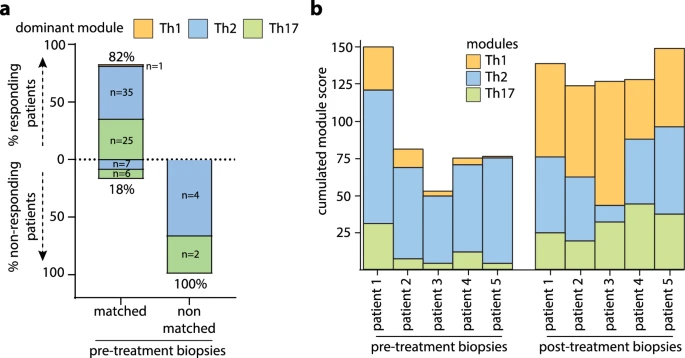 |
| Module matching to treatment targets to enhance therapeutic efficacy |
A blog that publishes updates and open access scientific papers about allergy, asthma and immunology. Editor: Juan Carlos Ivancevich, MD. Specialist in Allergy & Immunology
Seremet, T., Di Domizio, J., Girardin, A. et al. Nat Commun 15, 10688 (2024). https://doi.org/10.1038/s41467-024-54559-6
Abstract
 |
| Module matching to treatment targets to enhance therapeutic efficacy |
Qi, C., Li, A., Su, F. et al. Commun Biol 7, 1696 (2024). https://doi.org/10.1038/s42003-024-07416-7
Abstract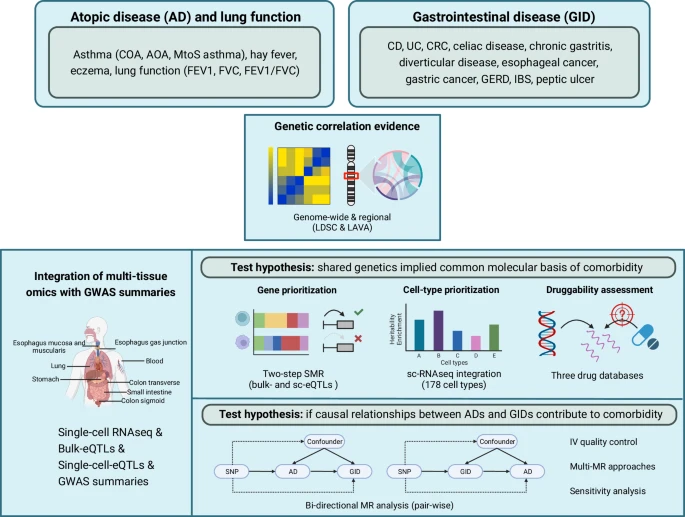 |
| Schematic visualization of the study workflow |
Abstract
Background: The decision whether to de-label patient with suspected BL hypersensitivity is based on risk stratification. The aim of this study was to prepare a characteristic of diagnostic risk groups and to create a model enabling the identification of the low-risk diagnostic group. Methods: We analyzed the medical records of patients hospitalized due to suspected hypersensitivity to BL antibiotics. Based on their medical-history data, patients were divided into three diagnostic risk groups, using the criteria proposed by Shenoy et al. Univariate and multivariate analysis models were used to create a diagnostic tool.
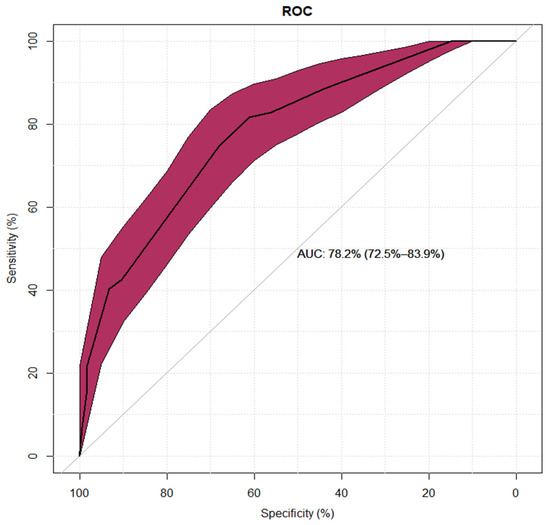 |
| Sensitivity and specificity for the multivariate logistic regression model of the low-diagnostic-risk group shown as a ROC (Receiver Operating Characteristic) curve. |
Malaya E, Piątkowska A, Panek M, Kuna P, Kupczyk M, Kardas G. Front Pharmacol. 2024 Dec 2;15:1488665. doi: 10.3389/fphar.2024.1488665.
Abstract
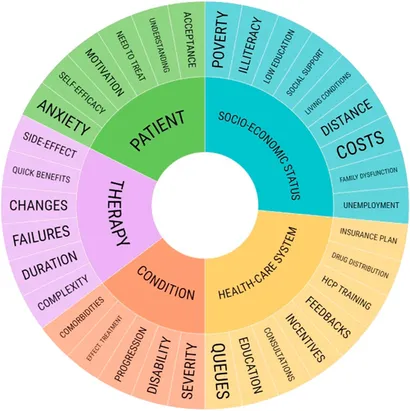 |
| 5 Dimensions affecting adherence. |
Schuster A, Caimmi D, Nolte H et al. Lancet Reg Health Eur. 2024 Nov 26;48:101136. doi: 10.1016/j.lanepe.2024.101136.
Abstract
Background: Allergic rhinitis/rhinoconjunctivitis (AR/C) induced by house dust mites (HDM) often begins in childhood and negatively impacts a child's quality of life. The daily burden can be further compounded by comorbid asthma. Allergen immunotherapy is the only available treatment targeting the underlying cause of allergic disease. Efficacy and safety of the SQ HDM sublingual immunotherapy (SLIT)-tablet has been demonstrated in adults and adolescents with HDM AR/C with or without asthma, but data are lacking for younger children.
Methods: Phase III, randomised, double-blind, placebo-controlled trial in younger children (5-11 years) with HDM AR/C with or without asthma. Eligible subjects were randomised 1:1 to SQ HDM SLIT-tablet or placebo for ∼1 year and had free access to AR/C symptom-relieving medications. The primary outcome was the total combined rhinitis score (TCRS) during the final 8 weeks of the treatment period (∼1 year). Secondary outcomes included the rhinitis daily symptom score (DSS) and medication score (DMS), the rhinoconjunctivitis total combined score (TCS), and the Paediatric Rhinoconjunctivitis Quality of Life Questionnaire (PRQLQ) score.
Boursiquot, JN., Gagnon, R., Quirt, J. et al. Allergy Asthma Clin Immunol 20 (Suppl 3), 66 (2024). https://doi.org/10.1186/s13223-024-00935-2
Allergen immunotherapy (AIT) is a potentially disease-modifying therapy that is effective for the treatment of allergic rhinitis/conjunctivitis, allergic asthma and stinging insect hypersensitivity. The decision to proceed with AIT should be made on a case-by-case basis, based on a comprehensive evaluation of the patient, allergy testing and a thorough discussion with the patient about treatment goals, risks vs. benefits, and long-term commitment to the treatment plan. For those with allergic rhinitis and/or asthma, it is also important to consider individual patient factors, such as the degree to which symptoms can be reduced by avoidance measures and pharmacological therapy, the amount and type of medication required to control symptoms, the adverse effects of pharmacological treatment, and patient preferences.
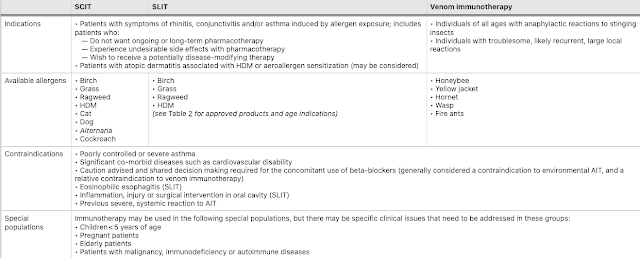 |
Since AIT is associated with a risk of anaphylaxis, it should only be prescribed by physicians who are adequately trained in the treatment of allergic conditions.
Jean Bousquet, Ludger Klimek, Hans-Christian Kuhl, Duc Tung Nguyen, Rajesh Kumar Ramalingam, G. Walter Canonica, William Berger; Int Arch Allergy Immunol 2024; https://doi.org/10.1159/000542054
Abstract
Background: Allergic rhinitis (AR) affects up to 40% of the pediatric population. The US practice parameter recommends the use of INAH or INCS as first-line therapy for the treatment of AR. Although not directly targeted to children, the recent US Practice Parameters proposed intranasal antihistamines as first-line therapy whereas the ARIA guidelines did not. Methods: This was a randomized, double-blind, parallel-group study with a duration of 28 days. It compared Azelastine hydrochloride 0.10% and 0.15% to placebo of one spray per nostril twice daily in pediatric subjects with moderate-to-severe symptomatic perennial allergic rhinitis (PAR).
Results: A total of 486 subjects were included in the study. The change from baseline rTNSS was statistically significant for 0.15% AZE (P = .005) and 0.10% AZE (P = .015) vs. placebo.Kim CK, Hwang Y, Song DJ, Yu J, Sohn MH, Park YM, Lim DH, Ahn K, Rha YH. Allergy Asthma Immunol Res. 2024 Nov;16(6):652-667. https://doi.org/10.4168/aair.2024.16.6.652
Abstract
Purpose
The combination therapy of leukotriene receptor antagonists and antihistamines may alleviate allergic rhinitis (AR) symptoms better than monotherapy. This study aimed to investigate the safety and efficacy of Monterizine®, a fixed-dose combination of montelukast and levocetirizine, compared to montelukast monotherapy in pediatric patients with AR.
Methods
One hundred seventy-six children aged 6 to 14 years with perennial AR symptoms were recruited. One hundred forty-seven subjects were randomized into 1 of 2 groups: the mont+levo group (fixed-dose combination of montelukast [5 mg] + levocetirizine [5 mg]) or the mont group (montelukast single agent [5 mg]).