Fan, X., Z. Liu, W. Yang, et al. 2025. Phytotherapy Research 1–30. https://doi.org/10.1002/ptr.70056.
ABSTRACT
 |
| Graphical Abstract |
A blog that publishes updates and open access scientific papers about allergy, asthma and immunology. Editor: Juan Carlos Ivancevich, MD. Specialist in Allergy & Immunology
Fan, X., Z. Liu, W. Yang, et al. 2025. Phytotherapy Research 1–30. https://doi.org/10.1002/ptr.70056.
 |
| Graphical Abstract |
Background and Aim: Coronavirus disease of 2019 (COVID-19), also known as SARS Coronavirus-2, is an infectious disease caused by a single-stranded RNA (ssRNA) virus that emerged in Wuhan, China, in December 2019, leading to a global pandemic. Among the vaccines developed for COVID-19, BioNTech, CoronaVac, and TURKOVAC™ have been administered in Türkiye. While they were the best way to control the pandemic, allergic reactions associated with these vaccines have been reported. We aimed to evaluate the possible risk factors by examining the demographic and clinical characteristics of patients who showed hypersensitivity reactions to BioNTech and CoronaVac vaccines, and especially any concomitant diseases. TURKOVAC™ was not administered to any of the patients who presented to our clinic.
Materials and Methods: This retrospective study included 45 patients who presented with hypersensitivity reactions to COVID-19 vaccines at the Istanbul Faculty of Medicine`s adult allergy clinic.
ABSTRACT
Statistical analysis plays a critical role in biomedical research, ensuring that data are interpreted appropriately and that conclusions are both valid and reproducible. In allergy and immunology, where studies increasingly rely on complex data structures and analytical approaches, clarity on biostatistical methods is essential to support transparency and scientific rigor. However, inconsistent statistical reporting and misuse of analytical techniques remain persistent challenges in the field. This review provides a structured and practice-oriented overview of key statistical aspects relevant to research in allergy and immunology.
Sabouraud-Leclerc, D., Mariotte, D., Bradatan, E., Divaret-Chauveau, A., Metz-Favre, C., Beaumont, P., Dumond, P., Serrier, J., Karaca-Altintas, Y., Tscheiller, S., Pouessel, G. and Van der Brempt, X. (2025), Clin Exp Allergy. https://doi.org/10.1111/cea.70130ABSTRACT
Abstract
Background
Since 2015, our nasal allergen challenge (NAC) protocol has been used to investigate the pathophysiology of allergic rhinitis (AR) with various allergens. However, we have yet to publish a comprehensive examination of the pathophysiology associated with AR to ragweed pollen.
Methods
Nineteen ragweed pollen allergic and 12 healthy (nonallergic) control participants from Kingston, Ontario, Canada, completed the NAC to ragweed pollen extract out-of-season. Total nasal symptom score (TNSS) and percent fall in peak nasal inspiratory flow (PNIF) were collected up to 48 h post-exposure. Nasal fluid and serum samples were collected post-exposure, and white blood cell differential counts, serum ragweed-specific and total immunoglobulin-E (IgE), and nasal cytokine concentrations were analyzed. Statistical tests were performed using GraphPad Prism 10.4.0.
Results
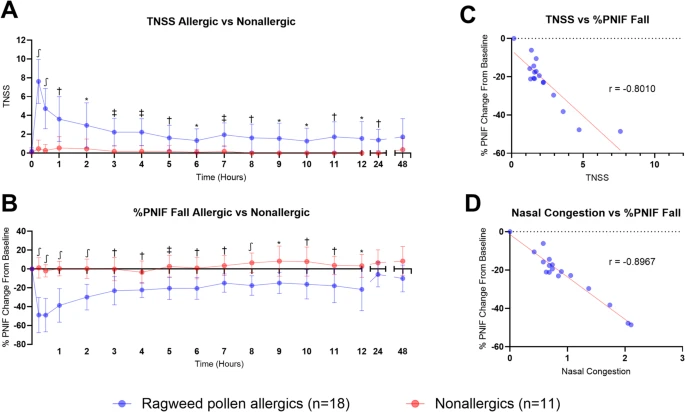 |
| Clinical symptoms induced by nasal allergen challenge (NAC) with Ragweed pollen |
Văruț RM, Dalia D, Radivojevic K, Trasca DM, Stoica GA, Adrian NS, Carmen NE, Singer CE. Pharmaceuticals (Basel). 2025 Jul 10;18(7):1021. doi: 10.3390/ph18071021.
Abstract
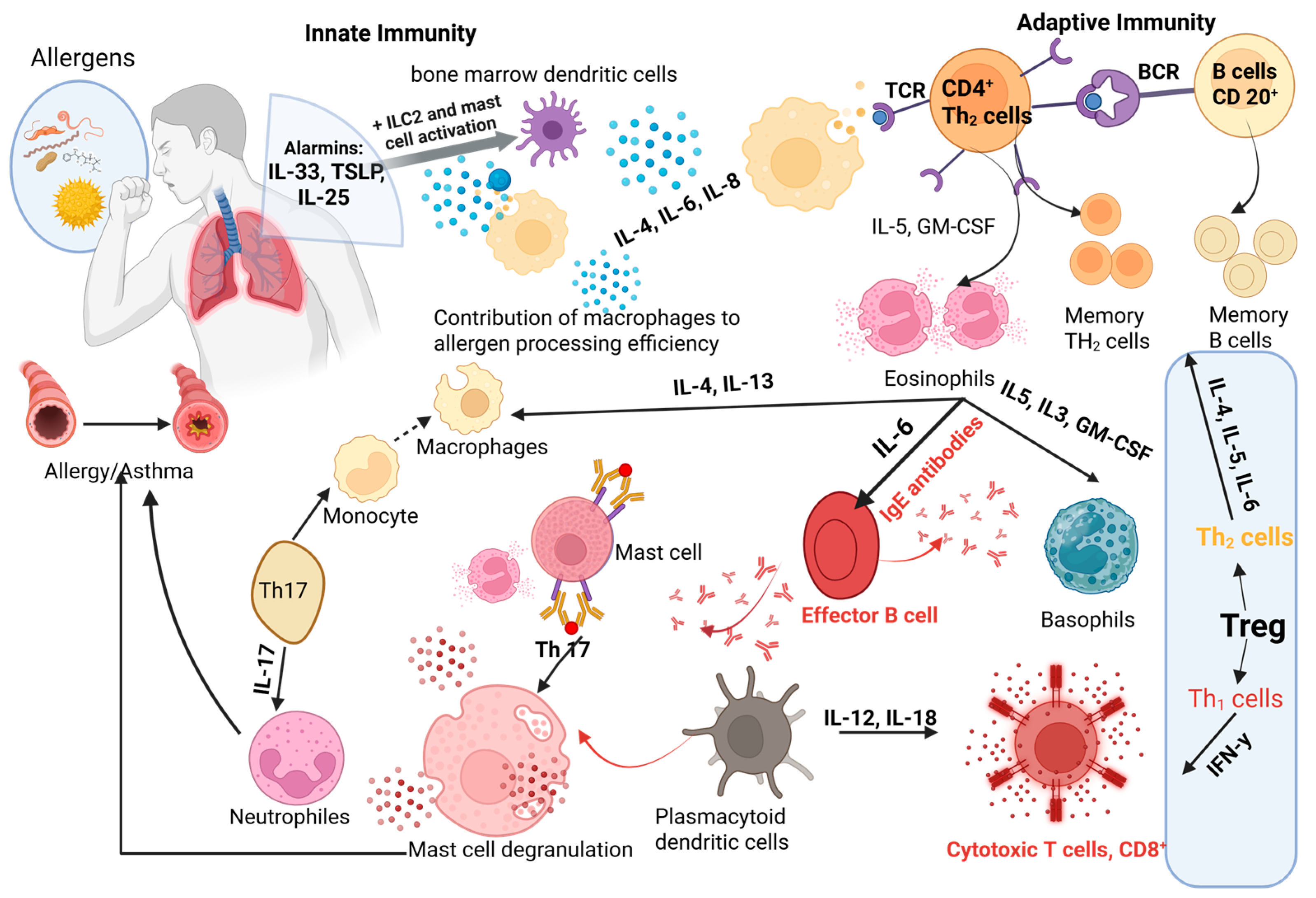 |
| Immune cell differentiation and IgE-mediated responses in allergic asthma pathogenesis |
Olbrich H, Preuß SL, Kridin K, Hernandez G, Thaçi D, Ludwig RJ, Curman P. J Allergy Clin Immunol. 2025 Aug 12:S0091-6749(25)00858-9. doi: 10.1016/j.jaci.2025.07.030.
ABSTRACT
Background
COVID-19 infection and vaccination have unclear impacts on type-2 inflammatory diseases. Although viral infections can drive immune dysregulation, the extent to which COVID-19 infection and vaccination affect type-2 inflammatory diseases in various organ systems remains underexplored.
Objective
We aimed to assess the risk of new-onset type-2 inflammatory diseases after COVID-19 infection and vaccination.
Methods
 |
| Flow-chart depicting the study outline. |
Copaescu, A.M., Chua, K.Y.L., Mouhtouris, E. et al. Allergy Asthma Clin Immunol 21, 35 (2025). https://doi.org/10.1186/s13223-025-00982-3
Abstract
Background
The use of in vivo and ex vivo diagnostic tools for delayed hypersensitivity reactions (DHRs) associated with iodinated contrast media (ICM) is currently ill-defined.
Objective
To evaluate the role of in vivo and ex vivo diagnostic tools for DHRs occurring >6 h following intravenous low-osmolality ICM.
Methods
We conducted a prospective, multicenter, international cohort study. The patients were recruited from two tertiary care adult allergy clinics, Austin Health, Australia and the McGill University Health Centre, Canada. Eligible participants were adults who reported a DHR after receiving ICM. In vivo testing (skin testing and intravenous challenge) was performed to identify an alternative agent. Ex vivo testing using interferon-γ enzyme-linked ImmunoSpot assay was performed in four Australian patients to explore its diagnostic performance.
Results
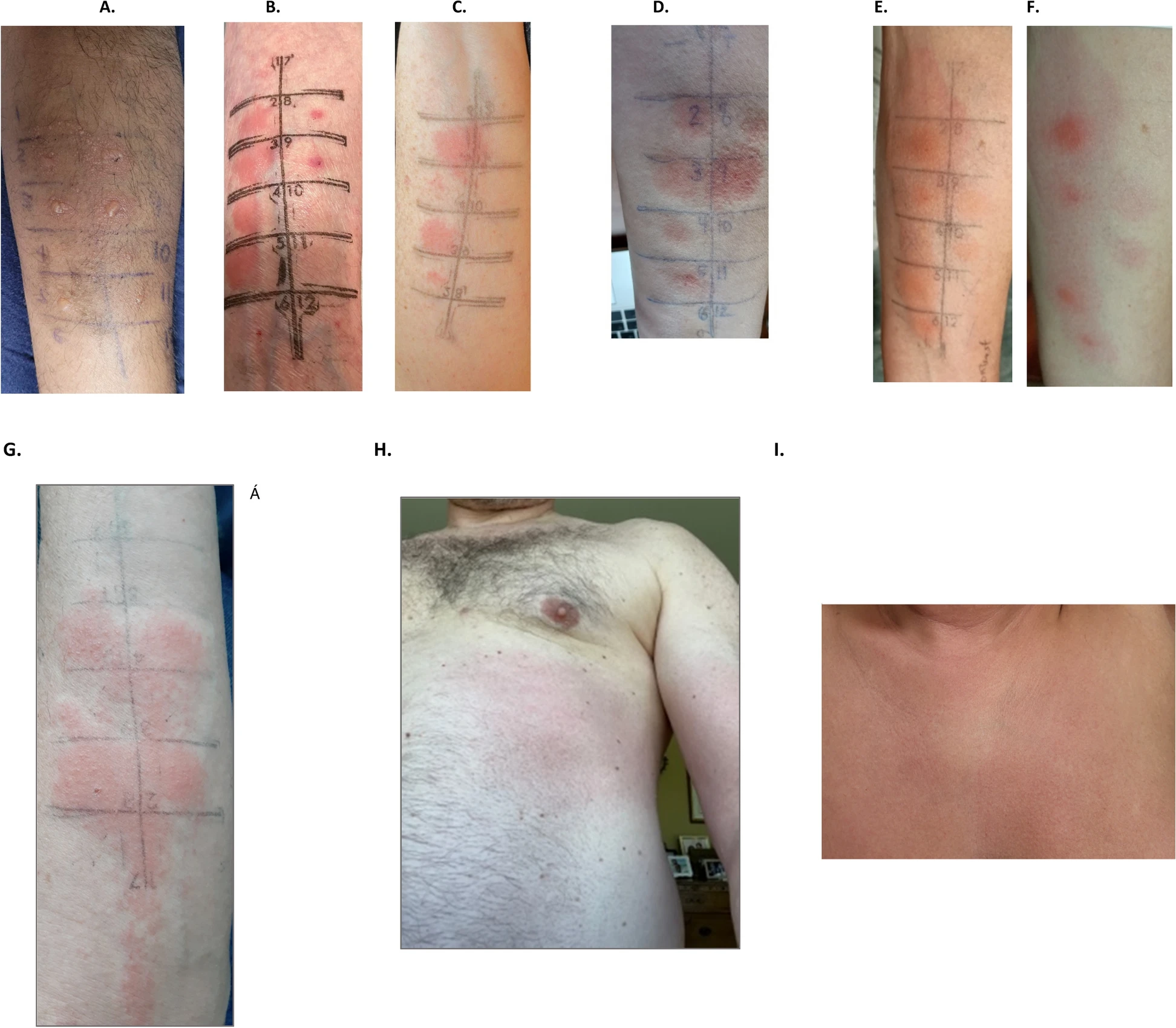 |
| Examples of delayed positive intradermal skin testing and intravenous challenges |