Kuna P, Jutel M, Pulka G, Tokarski S, Arranz P, Hernández G, Fernández Hernando N. Clin Ophthalmol. 2023 Mar 4;17:735-746. doi: 10.2147/OPTH.S398168.
Purpose: The objective of this study was to assess the safety and tolerability of preservative-free bilastine 0.6% ophthalmic solution after 8 weeks of once-daily administration in patients with allergic conjunctivitis (AC).
Patients and Methods: Multi-center, international, randomized, double blind, placebo-controlled, parallel-group, phase III study of adult patients with seasonal or perennial AC. The study was conducted in 26 centers of 5 European countries. Duration of daily treatment with bilastine 0.6% ophthalmic solution or placebo was 8 weeks.
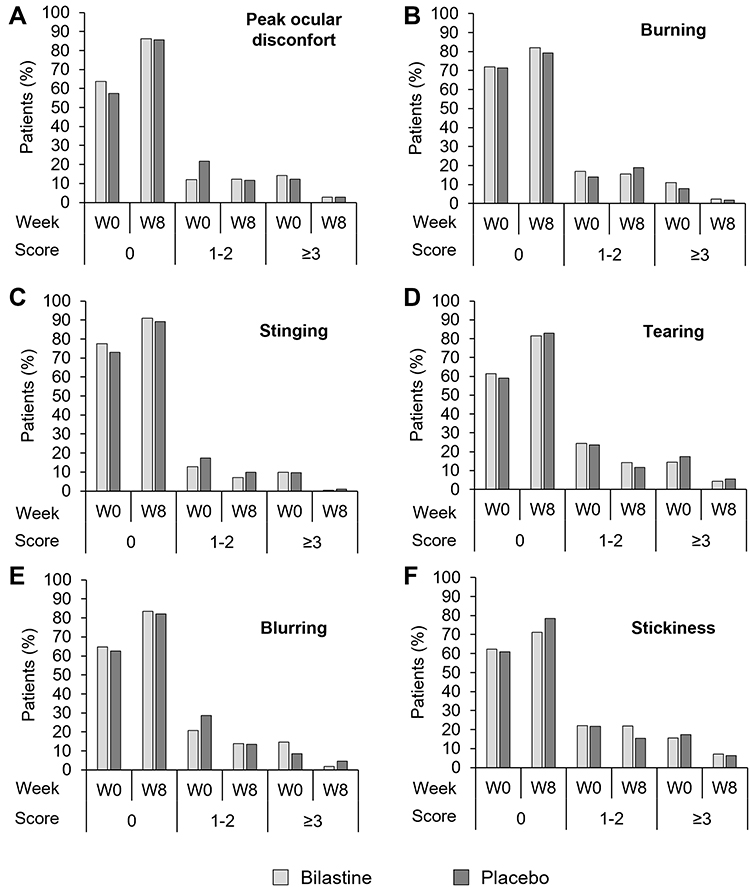
Safety was evaluated by analyzing incidence of ocular treatment-emergent adverse events (TEAEs); additionally, and as secondary parameters, ocular tolerability was assessed, in addition efficacy was also assessed by the average daily total eye symptoms score (TESS).
Conclusion: Bilastine 0.6% ophthalmic solution revealed no safety concerns in patients with AC after 8 weeks of once-daily administration. Bilastine was effective in reducing ocular symptoms associated with AC in response to both seasonal and perennial allergens.

No comments:
Post a Comment